The temporal transcriptomic signature of cartilage formation
- PMID: 36987858
- PMCID: PMC10164575
- DOI: 10.1093/nar/gkad210
The temporal transcriptomic signature of cartilage formation
Abstract
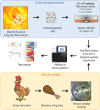
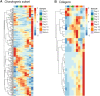
Chondrogenesis is a multistep process, in which cartilage progenitor cells generate a tissue with distinct structural and functional properties. Although several approaches to cartilage regeneration rely on the differentiation of implanted progenitor cells, the temporal transcriptomic landscape of in vitro chondrogenesis in different models has not been reported. Using RNA sequencing, we examined differences in gene expression patterns during cartilage formation in micromass cultures of embryonic limb bud-derived progenitors. Principal component and trajectory analyses revealed a progressively different and distinct transcriptome during chondrogenesis. Differentially expressed genes (DEGs), based on pairwise comparisons of samples from consecutive days were classified into clusters and analysed. We confirmed the involvement of the top DEGs in chondrogenic differentiation using pathway analysis and identified several chondrogenesis-associated transcription factors and collagen subtypes that were not previously linked to cartilage formation. Transient gene silencing of ATOH8 or EBF1 on day 0 attenuated chondrogenesis by deregulating the expression of key osteochondrogenic marker genes in micromass cultures. These results provide detailed insight into the molecular mechanism of chondrogenesis in primary micromass cultures and present a comprehensive dataset of the temporal transcriptomic landscape of chondrogenesis, which may serve as a platform for new molecular approaches in cartilage tissue engineering.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures











References
-
- Eyre D.R., Weis M.A., Wu J.J.. Articular cartilage collagen: an irreplaceable framework?. Eur. Cell Mater. 2006; 12:57–63. - PubMed
-
- Bhosale A.M., Richardson J.B.. Articular cartilage: structure, injuries and review of management. Br. Med. Bull. 2008; 87:77–95. - PubMed
-
- Hunter D.J., Bierma-Zeinstra S.. Osteoarthritis. Lancet. 2019; 393:1745–1759. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

