Cholinergic depolarization recruits a persistent Ca2+ current in Aplysia bag cell neurons
- PMID: 36988203
- PMCID: PMC11918274
- DOI: 10.1152/jn.00429.2022
Cholinergic depolarization recruits a persistent Ca2+ current in Aplysia bag cell neurons
Abstract
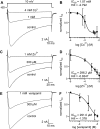
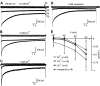
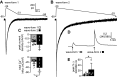
Many behaviors and types of information storage are mediated by lengthy changes in neuronal activity. In bag cell neurons of the hermaphroditic sea snail Aplysia californica, a transient cholinergic synaptic input triggers an ∼30-min afterdischarge. This causes these neuroendocrine cells to release egg laying hormone and elicit reproductive behavior. When acetylcholine is pressure-ejected onto a current-clamped bag cell neuron, the evoked depolarization is far longer than the current evoked by acetylcholine under voltage clamp, suggesting recruitment of another conductance. Our earlier studies found bag cell neurons to display a voltage-dependent persistent Ca2+ current. Hence, we hypothesized that this current is activated by the acetylcholine-induced depolarization and sought a selective Ca2+ current blocker. Rapid Ca2+ current evoked by 200-ms depolarizing steps in voltage-clamped cultured bag cell neurons demonstrated a concentration-dependent sensitivity to Ni2+, Co2+, Zn2+, and verapamil but not Cd2+ or ω-conotoxin GIVa. Leak subtraction of Ca2+ current evoked by 10-s depolarizing steps using the IC100 (concentration required to eliminate maximal current) of Ni2+, Co2+, Zn2+, or verapamil revealed persistent Ca2+ current, demonstrating persistent current block. Only Co2+ and Zn2+ did not suppress the acetylcholine-induced current, although Zn2+ appeared to impact additional channels. When Co2+ was applied during an acetylcholine-induced depolarization, the amplitude was reduced; furthermore, protein kinase C activation, previously established to enhance the persistent Ca2+ current, extended the depolarization. Therefore, the persistent Ca2+ current sustains the acetylcholine-induced depolarization and may translate brief cholinergic input into afterdischarge initiation. This could be a general mechanism of triggering long-term change in activity with a short-lived input.NEW & NOTEWORTHY Ionotropic acetylcholine receptors mediate brief synaptic communication, including in bag cell neurons of the sea snail Aplysia. However, this study demonstrates that cholinergic depolarization can open a voltage-gated persistent Ca2+ current, which extends the bag cell neuron response to acetylcholine. Bursting in these neuroendocrine cells results in hormone release and egg laying. Thus, this emphasizes the role of ionotropic signaling in reaching a depolarized level to engage Ca2+ influx and perpetuating the activity necessary for behavior.
Keywords: acetylcholine receptor; mollusk; peptidergic neuron; prolonged depolarization; reproduction.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

