A network meta-analysis of the efficacy of hypoxia-inducible factor prolyl-hydroxylase inhibitors in dialysis chronic kidney disease
- PMID: 36988549
- PMCID: PMC10085583
- DOI: 10.18632/aging.204611
A network meta-analysis of the efficacy of hypoxia-inducible factor prolyl-hydroxylase inhibitors in dialysis chronic kidney disease
Abstract
Background: Five types of HIF-PHIs have been authorized for anemia treatment in CKD patients in China and Japan. These are enarodustat, roxadustat, daprodustat, vadadustat, and molidustat. How effectively they compare to ESAs about clinical results in CKD-DD patients is uncertain. This study examined the RCT evidence about the benefits and risks of HIF-PHIs and ESAs in dialysis CKD patients.
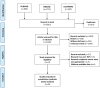
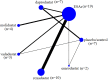
Methods: We conducted an extensive investigation and network meta-analysis of RCTs. In these RCTs, patients with CKD-DD received one of five different HIF-PHI or ESAs, a placebo, and no medical intervention. Outcomes included hemoglobin, iron parameters, and adverse events, and there were four weeks of follow-up at least. A frequentist framework for multivariate random effects meta-analyzed the results. The effect sizes of categorical variables were displayed as odds ratios. Mean differences were employed for computing continuous outcomes with common units; otherwise, standardized mean differences were applied. The Cochrane tool evaluated the bias risk in RCTs.
Results: 26 RCTs with 14945 patients were qualified for inclusion. Compared to the placebo, HIF-PHIs and ESAs dramatically boosted hemoglobin without affecting serum iron. Roxadustat performed better hemoglobin levels than ESAs (MD 0.32, 95% CI 0.10 to 0.53) and daprodustat (0.46, 0.09 to 0.84). Roxadustat (91.8%) was the top hemoglobin treatment among all medical interventions, as determined by the SUCRA ranking. However, roxadustat caused more thrombosis and hypertension than ESAs (1.61, 1.22 to 2.12) and vadadustat (1.36, 1.01 to 1.82). The lowest rates of hypertension and thrombosis were seen in molidustat (80.7%) and ESAs (88.5%). Compared with a placebo, ESAs and HIF-PHIs all affected TSAT levels. Except for molidustat, the other four HIF-PHIs impact different iron parameters. Regarding ferritin reduction, roxadustat (90.9%) and daprodustat (60.9%) came out on top. Enarodustat (80.9%) and roxadustat (74%) placed best and second in lowering hepcidin levels. The former two medicines for TIBC improvement were vadadustat (98.7%) and enarodustat (80.9%).
Conclusion: The most effective treatment for hemoglobin correction is roxadustat. The superior efficacy of reducing hepcidin makes roxadustat and enarodustat appropriate for patients with inflammation. However, the increased risk of hypertension and thrombosis associated with roxadustat should be noted. In patients at risk for hypertension and thrombosis, molidustat and ESAs may be preferable options. When administering roxadustat and daprodustat, clinicians should check ferritin to assess iron storage. Lower TSAT in patients receiving HIF-PHIs and ESAs treatment suggests intravenous iron supplements are needed.
Keywords: anemia; chronic kidney disease; dialysis; erythropoiesis-stimulating agents; hypoxia-inducible factor prolyl-hydroxylase inhibitors.
Conflict of interest statement
Figures



References
-
- Mcmurray JJV, Parfrey PS, Adamson JW, Aljama P, Berns JS, Bohlius J, Drüeke TB, Finkelstein FO, Fishbane S, Ganz T, Macdougall I, McDonald RA, Mcmahon L, et al.. Kidney disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012; 2:279–335. 10.1038/kisup.2012.37 - DOI
-
- National Clinical Guideline Centre. Anaemia management in chronic kidney disease: update 2015 NICE guideline 8. National Institute for Health and Care Excellence (NICE). 2019. https://www.nice.org.uk/guidance/ng8
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

