A linear SARS-CoV-2 DNA vaccine candidate reduces virus shedding in ferrets
- PMID: 36988739
- PMCID: PMC10052258
- DOI: 10.1007/s00705-023-05746-1
A linear SARS-CoV-2 DNA vaccine candidate reduces virus shedding in ferrets
Abstract
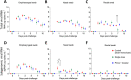
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), has caused more than 760 million cases and over 6.8 million deaths as of March 2023. Vaccination has been the main strategy used to contain the spread of the virus and to prevent hospitalizations and deaths. Currently, two mRNA-based vaccines and one adenovirus-vectored vaccine have been approved and are available for use in the U.S. population. The versatility, low cost, and rapid production of DNA vaccines provide important advantages over other platforms. Additionally, DNA vaccines efficiently induce both B- and T-cell responses by expressing the antigen within transfected host cells, and the antigen, after being processed into peptides, can associate with MHC class I or II of antigen-presenting cells (APCs) to stimulate different T cell responses. However, the efficiency of DNA vaccination needs to be improved for use in humans. Importantly, in vivo DNA delivery combined with electroporation (EP) has been used successfully in the field of veterinary oncology, resulting in high rates of response after electrochemotherapy. Here, we evaluate the safety, immunogenicity, and protective efficacy of a novel linear SARS-CoV-2 DNA vaccine candidate delivered by intramuscular injection followed by electroporation (Vet-ePorator™) in ferrets. The linear SARS-CoV-2 DNA vaccine candidate did not cause unexpected side effects. Additionally, the vaccine elicited neutralizing antibodies and T cell responses on day 42 post-immunization using a low dose of the linear DNA construct in a prime-boost regimen. Most importantly, vaccination significantly reduced shedding of infectious SARS-CoV-2 through oral and nasal secretions in a ferret model.
© 2023. The Author(s), under exclusive licence to Springer-Verlag GmbH Austria, part of Springer Nature.
Conflict of interest statement
A.C. is an Evvivax employee. L.L., E.S., and L.A. are Takis employees. Takis and Rottapharm Biotech are jointly developing COVID-eVax. B.V., J.H., and C.S. are employees of Applied DNA Sciences and LineaRx, which retain the IP rights for the linear DNA platform.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

