Intravascularly Deliverable Biomaterial Platforms for Tissue Repair and Regeneration Post-Myocardial Infarction
- PMID: 36989469
- PMCID: PMC10539487
- DOI: 10.1002/adma.202300603
Intravascularly Deliverable Biomaterial Platforms for Tissue Repair and Regeneration Post-Myocardial Infarction
Abstract
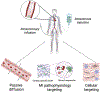
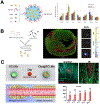
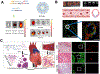
Each year, nearly 19 million people die of cardiovascular disease with coronary heart disease and myocardial infarction (MI) as the leading cause of the progression of heart failure. Due to the high risk associated with surgical procedures, a variety of minimally invasive therapeutics aimed at tissue repair and regeneration are being developed. While biomaterials delivered via intramyocardial injection have shown promise, there are challenges associated with delivery in acute MI. In contrast, intravascularly injectable biomaterials are a desirable category of therapeutics due to their ability to be delivered immediately post-MI via less invasive methods. In addition to passive diffusion into the infarct, these biomaterials can be designed to target the molecular and cellular characteristics seen in MI pathophysiology, such as cells and proteins present in the ischemic myocardium, to reduce off-target localization. These injectable materials can also be stimuli-responsive through enzymes or chemical imbalances. This review outlines the natural and synthetic biomaterial designs that allow for retention and accumulation within the infarct via intravascular delivery, including intracoronary infusion and intravenous injection.
Keywords: cardiac repair; injectable biomaterials; intravascular biomaterials; myocardial infarction.
© 2023 Wiley‐VCH GmbH.
Figures


References
-
- Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Ferguson JF, Generoso G, Ho JE, Kalani R, Khan SS, Kissela BM, Knutson KL, Levine DA, Lewis TT, Liu J, Loop MS, Ma J, Mussolino ME, Navaneethan SD, Perak AM, Poudel R, Rezk-Hanna M, Roth GA, Schroeder EB, Shah SH, Thacker EL, VanWagner LB, Virani SS, Voecks JH, Wang N-Y, Yaffe K, Martin SS, Circulation 2022, 145, e153. - PubMed
-
- Lu L, Liu M, Sun R, Zheng Y, Zhang P, Cell Biochem Biophys 2015, 72, 865. - PubMed
-
- Frangogiannis NG, Smith CW, Entman ML, Cardiovascular Research 2002, 53, 31. - PubMed
-
- Tous E, Purcell B, Ifkovits JL, Burdick JA, J Cardiovasc Transl Res 2011, 4, 528. - PubMed
-
- Johnson TD, Christman KL, Expert opinion on drug delivery 2013, 10, 59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

