A multi-country phase 2 study to evaluate the suitcase lab for rapid detection of SARS-CoV-2 in seven Sub-Saharan African countries: Lessons from the field
- PMID: 36989731
- PMCID: PMC9981265
- DOI: 10.1016/j.jcv.2023.105422
A multi-country phase 2 study to evaluate the suitcase lab for rapid detection of SARS-CoV-2 in seven Sub-Saharan African countries: Lessons from the field
Abstract
Background: The COVID-19 pandemic led to severe health systems collapse, as well as logistics and supply delivery shortages across sectors. Delivery of PCR related healthcare supplies continue to be hindered. There is the need for a rapid and accessible SARS-CoV-2 molecular detection method in low resource settings.
Objectives: To validate a novel isothermal amplification method for rapid detection of SARS-CoV-2 across seven sub-Sharan African countries.
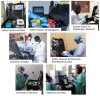
Study design: In this multi-country phase 2 diagnostic study, 3,231 clinical samples in seven African sites were tested with two reverse transcription Recombinase-Aided Amplification (RT-RAA) assays (based on SARS-CoV-2 Nucleocapsid (N) gene and RNA-dependent RNA polymerase (RdRP) gene). The test was performed in a mobile suitcase laboratory within 15 min. All results were compared to a real-time RT-PCR assay. Extraction kits based on silica gel or magnetic beads were applied.
Results: Four sites demonstrated good to excellent agreement, while three sites showed fair to moderate results. The RdRP gene assay exhibited an overall PPV of 0.92 and a NPV of 0.88. The N gene assay exhibited an overall PPV of 0.93 and a NPV 0.88. The sensitivity of both RT-RAA assays varied depending on the sample Ct values. When comparing sensitivity between sites, values differed considerably. For high viral load samples, the RT-RAA assay sensitivity ranges were between 60.5 and 100% (RdRP assay) and 25 and 98.6 (N assay).
Conclusion: Overall, the RdRP based RT-RAA test showed the best assay accuracy. This study highlights the challenges of implementing rapid molecular assays in field conditions. Factors that are important for successful deployment across countries include the implementation of standardized operation procedures, in-person continuous training for staff, and enhanced quality control measures.
Keywords: Diagnostics-in-a-suitcase; Recombinase polymerase amplification assay; SARS-CoV-2.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Organization, W.H. WHO announces COVID-19 outbreak a pandemic. 2020.
-
- Zhu G., Chou M.C., Tsai C.W. Lessons learned from the COVID-19 pandemic exposing the shortcomings of current supply chain operations: a long-term prescriptive offering. Sustainability. 2020;12:5858.
-
- Iyengar K.P., Vaishya R., Bahl S., Vaish A. Impact of the coronavirus pandemic on the supply chain in healthcare. British J. Healthcare Manag. 2020;26:1–4.
-
- Organization W.H. World Health Organization; 2021. Antigen-Detection in the Diagnosis of SARS-CoV-2 Infection: Interim Guidance, 6 October 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

