Identification of HPV16 E1 and E2-specific T cells in the oropharyngeal cancer tumor microenvironment
- PMID: 36990508
- PMCID: PMC10069587
- DOI: 10.1136/jitc-2023-006721
Identification of HPV16 E1 and E2-specific T cells in the oropharyngeal cancer tumor microenvironment
Abstract
Background: High-risk human papillomavirus (HPV) is a primary cause of an increasing number of oropharyngeal squamous cell carcinomas (OPSCCs). The viral etiology of these cancers provides the opportunity for antigen-directed therapies that are restricted in scope compared with cancers without viral components. However, specific virally-encoded epitopes and their corresponding immune responses are not fully defined.
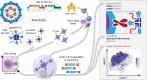
Methods: To understand the OPSCC immune landscape, we conducted a comprehensive single-cell analysis of HPV16+ and HPV33+ primary tumors and metastatic lymph nodes. We used single-cell analysis with encoded peptide-human leukocyte antigen (HLA) tetramers to analyze HPV16+ and HPV33+ OPSCC tumors, characterizing the ex vivo cellular responses to HPV-derived antigens presented in major Class I and Class II HLA alleles.
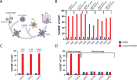
Results: We identified robust cytotoxic T-cell responses to HPV16 proteins E1 and E2 that were shared across multiple patients, particularly in HLA-A*01:01 and HLA-B*08:01. Responses to E2 were associated with loss of E2 expression in at least one tumor, indicating the functional capacity of these E2-recognizing T cells and many of these interactions validated in a functional assay. Conversely, cellular responses to E6 and E7 were limited in quantity and cytotoxic capacity, and tumor E6 and E7 expression persisted.
Conclusions: These data highlight antigenicity beyond HPV16 E6 and E7 and nominate candidates for antigen-directed therapies.
Keywords: Adaptive Immunity; Head and Neck Neoplasms; Lymphocytes, Tumor-Infiltrating; T-Lymphocytes; Tumor Microenvironment.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: CM, BV, QT, DL-E, DCP and AJC are employees and/or stockholders of Repertoire Immune Medicine. GJH reports grants and institutional support from ASCO CCF, Bicara, BMS, Gateway for Cancer Research, GSK, Kite, KSQ, Kura Oncology, ImmunityBio, Regeneron. Consulting/honoraria from Bicara, BMS, Coherus, Exicure, Kura, Maverick, Merck, Naveris, Regeneron, and SIRPant. AME reports support from NIH/NIDCR U0 DE029188. RU serves on a Merck advisory board.
Figures


References
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
