Feasibility of methotrexate discontinuation following tocilizumab and methotrexate combination therapy in patients with long-standing and advanced rheumatoid arthritis: a 3-year observational cohort study
- PMID: 36990790
- PMCID: PMC10122970
- DOI: 10.5387/fms.2022-06
Feasibility of methotrexate discontinuation following tocilizumab and methotrexate combination therapy in patients with long-standing and advanced rheumatoid arthritis: a 3-year observational cohort study
Retraction in
-
RETRACTED ARTICLE.Fukushima J Med Sci. 2024 Jan 27;70(1):55. doi: 10.5387/fms.retraction. Epub 2024 Jan 25. Fukushima J Med Sci. 2024. PMID: 38267028 Free PMC article. No abstract available.
Abstract
Objectives: Methotrexate (MTX) is associated with extensive side effects, including myelosuppression, interstitial pneumonia, and infection. It is, therefore, critical to establish whether its administration is required after achieving remission with tocilizumab (TCZ) and MTX combination therapy in patients with rheumatoid arthritis (RA). Therefore, the aim of this multicenter, observational, cohort study was to evaluate the feasibility of MTX discontinuation for the safety of these patients.
Methods: Patients with RA were administered TCZ, with or without MTX, for 3 years; those who received TCZ+MTX combination therapy were selected. After remission was achieved, MTX was discontinued without flare development in one group (discontinued [DISC] group, n = 33) and continued without flare development in another group (maintain [MAIN] group, n = 37). The clinical efficacy of TCZ+MTX therapy, patient background characteristics, and adverse events were compared between groups.
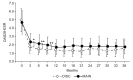
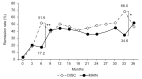
Results: The disease activity score in 28 joints-erythrocyte sedimentation rate (DAS28-ESR) at 3, 6, and 9 months was significantly lower in the DISC group (P < .05, P < .01, and P < .01, respectively). Further, the DAS28-ESR remission rate at 6 and 9 months and Boolean remission rate at 6 months were significantly higher in the DISC group (P < .01 for all). Disease duration was significantly longer in the DISC group (P < .05). Furthermore, the number of patients with stage 4 RA was significantly higher in the DISC group (P < .01).
Conclusions: Once remission was achieved, MTX was discontinued in patients who responded favorably to TCZ+MTX therapy, despite the prolonged disease duration and stage progression.
Keywords: discontinuation; long-standing; methotrexate; rheumatoid arthritis; tocilizumab.
Conflict of interest statement
The authors of this work have nothing to disclose.
Figures


References
-
- Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis, 79: 685-699, 2020. https://doi.org/10.1136/annrheumdis-2019-216655. - PubMed
-
- Kerschbaumer A, Sepriano A, Smolen JS, et al. Efficacy of pharmacological treatment in rheumatoid arthritis: A systematic literature research informing the 2019 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis, 79: 744-759, 2020. https://doi.org/10.1136/annrheumdis-2019-216656. - PMC - PubMed
-
- Nam JL, Winthrop KL, van Vollenhoven RF, et al. Current evidence for the management of rheumatoid arthritis with biological disease-modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of RA. Ann Rheum Dis, 69: 976-986, 2010. https://doi.org/10.1136/ard.2009.126573. - PubMed
-
- Silvagni E, Bortoluzzi A, Carrara G, Zanetti A, Govoni M, Scirè CA. Comparative effectiveness of first-line biological monotherapy use in rheumatoid arthritis: a retrospective analysis of the RECord-linkage On Rheumatic Diseases study on health care administrative database. BMJ Open. 2018; 8: e021447. http://dx.doi.org/10.1136/bmjopen-2017-021447. - PMC - PubMed
-
- Finzel S, Kraus S, Figueiredo CP, et al. Comparison of the effects of tocilizumab monotherapy and adalimumab in combination with methotrexate on bone erosion repair in rheumatoid arthritis. Ann Rheum Dis, 78: 1186-1191, 2019. https://doi.org/10.1136/annrheumdis-2018-214894. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

