Cessation of chronic delta-9-tetrahydrocannabinol use partially reverses impacts on male fertility and the sperm epigenome in rhesus macaques
- PMID: 36990913
- PMCID: PMC10293074
- DOI: 10.1016/j.fertnstert.2023.02.034
Cessation of chronic delta-9-tetrahydrocannabinol use partially reverses impacts on male fertility and the sperm epigenome in rhesus macaques
Abstract
Objective: To determine whether discontinuation of delta-9-tetrahydrocannabinol (THC) use mitigates THC-associated changes in male reproductive health using a rhesus macaque model of daily THC edible consumption.
Design: Research animal study.
Setting: Research institute environment.
Patient(s): Adult male rhesus macaques (age, 8-10 years; n = 6).
Intervention(s): Chronic daily THC edible administration at medically and recreationally relevant contemporary doses followed by cessation of THC use.
Main outcome measure(s): Testicular volume, serum male hormones, semen parameters, sperm deoxyribonucleic acid (DNA) fragmentation, seminal fluid proteomics, and whole genome bisulfite sequencing of sperm DNA.
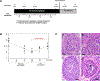
Result(s): Chronic THC use resulted in significant testicular atrophy, increased gonadotropin levels, decreased serum sex steroid levels, changes in seminal fluid proteome, and increased DNA fragmentation with partial recovery after discontinuation of THC use. For every increase of 1 mg/7 kg/day in THC dosing, there was a significant decrease in the total testicular volume bilaterally by 12.6 cm3 (95% confidence interval [CI], 10.6-14.5), resulting in a 59% decrease in volume. With THC abstinence, the total testicular volume increased to 73% of its original volume. Similarly, with THC exposure, there were significant decreases in the mean total testosterone and estradiol levels and a significant increase in the follicle-stimulating hormone level. With increasing THC dose, there was a significant decrease in the liquid semen ejaculate volume and weight of coagulum; however, no other significant changes in the other semen parameters were noted. After discontinuing THC use, there was a significant increase in the total serum testosterone level by 1.3 ng/mL (95% CI, 0.1-2.4) and estradiol level by 2.9 pg/mL (95% CI, 0.4-5.4), and the follicle-stimulating hormone level significantly decreased by 0.06 ng/mL (95% CI, 0.01-0.11). Seminal fluid proteome analysis revealed differential expression of proteins enriched for processes related to cellular secretion, immune response, and fibrinolysis. Whole genome bisulfite sequencing identified 23,558 CpGs differentially methylated in heavy-THC vs. pre-THC sperm, with partial restoration of methylation after discontinuation of THC use. Genes associated with altered differentially methylated regions were enriched for those involved in the development and function of the nervous system.
Conclusion(s): This is the first study demonstrating that discontinuation of chronic THC use in rhesus macaques partially restores adverse impacts to male reproductive health, THC-associated sperm differentially methylated regions in genes important for development, and expression of proteins important for male fertility.
Keywords: Male fertility; THC; cannabis; delta-9-tetrahydrocannabinol; marijuana.
Copyright © 2023 American Society for Reproductive Medicine. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


Comment in
-
Partial reversal of chronic delta-9-tetrahydrocannabinol-induced effects on male fertility and sperm epigenome after cessation.Fertil Steril. 2023 Jul;120(1):175. doi: 10.1016/j.fertnstert.2023.04.034. Epub 2023 Apr 29. Fertil Steril. 2023. PMID: 37121568 No abstract available.
References
-
- Center for Behavioral Health Statistics and Quality. Key substance use and mental health indicators in the United States: results from the 2019 National Survey on Drug Use and Health 2020.
-
- Optimizing natural fertility: a committee opinion. Fertil Steril 2022;117:53–63. - PubMed
-
- Dalterio S, Badr F, Bartke A, Mayfield D. Cannabinoids in male mice: effects on fertility and spermatogenesis. Science 1982;216:315–6. - PubMed
-
- Symons A, Teale J, Marks V. Proceedings: Effect of delta9-tetrahydrocannabinol on the hypothalamic-pituitary-gonadal system in the maturing male rat. The Journal of endocrinology 1976;68:43P–4P. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

