Self assembling nanoparticle enzyme clusters provide access to substrate channeling in multienzymatic cascades
- PMID: 36990995
- PMCID: PMC10060375
- DOI: 10.1038/s41467-023-37255-9
Self assembling nanoparticle enzyme clusters provide access to substrate channeling in multienzymatic cascades
Abstract
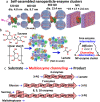
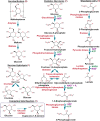
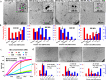
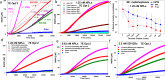
Access to efficient enzymatic channeling is desired for improving all manner of designer biocatalysis. We demonstrate that enzymes constituting a multistep cascade can self-assemble with nanoparticle scaffolds into nanoclusters that access substrate channeling and improve catalytic flux by orders of magnitude. Utilizing saccharification and glycolytic enzymes with quantum dots (QDs) as a model system, nanoclustered-cascades incorporating from 4 to 10 enzymatic steps are prototyped. Along with confirming channeling using classical experiments, its efficiency is enhanced several fold more by optimizing enzymatic stoichiometry with numerical simulations, switching from spherical QDs to 2-D planar nanoplatelets, and by ordering the enzyme assembly. Detailed analyses characterize assembly formation and clarify structure-function properties. For extended cascades with unfavorable kinetics, channeled activity is maintained by splitting at a critical step, purifying end-product from the upstream sub-cascade, and feeding it as a concentrated substrate to the downstream sub-cascade. Generalized applicability is verified by extending to assemblies incorporating other hard and soft nanoparticles. Such self-assembled biocatalytic nanoclusters offer many benefits towards enabling minimalist cell-free synthetic biology.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
I.L.M., J.N.V., M.G.A., K.S., and S.A.D. received Patent No.: US 11,512,305 B2 entitled Nanoparticle-Attached Enzyme Cascades for Accelerated Multistep Biocatalysis, which was filed by the US Navy. This patent includes some of the processes and phenomena described herein. The remaining authors declare no competing interests.
Figures


References
-
- Adesina O, Anzai IA, Avalos JL, Barstow B. Embracing biological solutions to the sustainable energy challenge. Chem. 2017;2:20–51.
-
- Lee SY, et al. A comprehensive metabolic map for production of bio-based chemicals. Nat. Catal. 2019;2:18–33.
-
- Keasling JD. Manufacturing molecules through metabolic engineering. Science. 2010;330:1355–1358. - PubMed
-
- Nielsen J, Keasling JD. Engineering cellular metabolism. Cell. 2016;164:1185–1197. - PubMed
-
- Clomburg, J. M., Crumbley, A. M. & Gonzalez, R. Industrial biomanufacturing: the future of chemical production. Science10.1126/science.aag0804 (2017). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

