Mechanical and leakage integrity testing considerations for evaluating the performance of tissue containment systems
- PMID: 36991010
- PMCID: PMC10060240
- DOI: 10.1038/s41598-023-31847-7
Mechanical and leakage integrity testing considerations for evaluating the performance of tissue containment systems
Abstract
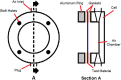
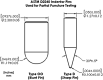
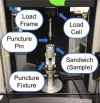
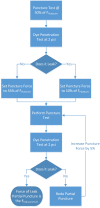
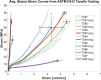
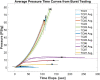
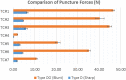
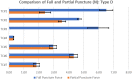
Tissue containment systems (TCS) are medical devices that may be used during morcellation procedures during minimally invasive laparoscopic surgery. TCS are not new devices but their use as a potential mitigation for the spread of occult malignancy during laparoscopic power morcellation of fibroids and/or the uterus has been the subject of interest following reports of upstaging of previously undetected sarcoma in women who underwent a laparoscopic hysterectomy. Development of standardized test methods and acceptance criteria to evaluate the safety and performance of these devices will speed development, allowing for more devices to benefit patients. As a part of this study, a series of preclinical experimental bench test methods were developed to evaluate the mechanical and leakage performance of TCS that may be used in power morcellation procedures. Experimental tests were developed to evaluate mechanical integrity, e.g., tensile, burst, puncture, and penetration strengths for the TCS, and leakage integrity, e.g., dye and microbiological leakage (both acting as surrogates for blood and cancer cells) through the TCS. In addition, to evaluate both mechanical integrity and leakage integrity as a combined methodology, partial puncture and dye leakage was conducted on the TCS to evaluate the potential for leakage due to partial damage caused by surgical tools. Samples from 7 different TCSs were subjected to preclinical bench testing to evaluate leakage and mechanical performance. The performance of the TCSs varied significantly between different brands. The leakage pressure of the TCS varied between 26 and > 1293 mmHg for the 7 TCS brands. Similarly, the tensile force to failure, burst pressure, and puncture force varied between 14 and 80 MPa, 2 and 78 psi, and 2.5 N and 47 N, respectively. The mechanical failure and leakage performance of the TCS were different for homogeneous and composite TCSs. The test methods reported in this study may facilitate the development and regulatory review of these devices, may help compare TCS performance between devices, and increase provider and patient accessibility to improved tissue containment technologies.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
In Vitro Leakage Testing of Tissue Containment Bags When Subjected to Power Morcellation Forces.J Minim Invasive Gynecol. 2020 Mar-Apr;27(3):655-664. doi: 10.1016/j.jmig.2019.05.006. Epub 2019 May 21. J Minim Invasive Gynecol. 2020. PMID: 31125722
-
Risk of leakage with a new detachable multi-hard-port containment system for power morcellation during gynecologic laparoscopy: An in vitro study.BMC Surg. 2023 Jul 31;23(1):213. doi: 10.1186/s12893-023-02124-1. BMC Surg. 2023. PMID: 37525186 Free PMC article.
-
Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters.Am J Obstet Gynecol. 2016 Feb;214(2):257.e1-257.e6. doi: 10.1016/j.ajog.2015.08.076. Epub 2015 Sep 6. Am J Obstet Gynecol. 2016. PMID: 26348384
-
In-bag manual versus uncontained power morcellation for laparoscopic myomectomy.Cochrane Database Syst Rev. 2020 May 6;5(5):CD013352. doi: 10.1002/14651858.CD013352.pub2. Cochrane Database Syst Rev. 2020. PMID: 32374421 Free PMC article.
-
Surgical Treatment of Uterine Fibroids Within a Containment System and Without Power Morcellation.Clin Obstet Gynecol. 2016 Mar;59(1):85-92. doi: 10.1097/GRF.0000000000000168. Clin Obstet Gynecol. 2016. PMID: 26670832 Review.
Cited by
-
Tailoring the Diagnostic Pathway for Medical and Surgical Treatment of Uterine Fibroids: A Narrative Review.Diagnostics (Basel). 2024 Sep 14;14(18):2046. doi: 10.3390/diagnostics14182046. Diagnostics (Basel). 2024. PMID: 39335725 Free PMC article. Review.
-
Exploring Surgical Strategies for Uterine Fibroid Treatment: A Comprehensive Review of Literature on Open and Minimally Invasive Approaches.Medicina (Kaunas). 2023 Dec 28;60(1):64. doi: 10.3390/medicina60010064. Medicina (Kaunas). 2023. PMID: 38256325 Free PMC article. Review.
-
Laparoscopic continuous seromuscular circumsuture for myomectomy: a real-world, retrospective, East-Asian cohort study.BMJ Open. 2024 Mar 8;14(3):e081550. doi: 10.1136/bmjopen-2023-081550. BMJ Open. 2024. PMID: 38458810 Free PMC article.
References
-
- US Food and Drug Administration. UPDATED laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. (Silver Spring, MD, 2014).
-
- US Government Accountability Office. MEDICAL DEVICES: Cancer risk led FDA to warn against certain uses of power morcellators and recommend new labeling. pp 49 (US Government Accountability Office, Washington, DC, 2017).
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

