Cell-autonomous effect of cardiomyocyte branched-chain amino acid catabolism in heart failure in mice
- PMID: 36991098
- PMCID: PMC10310802
- DOI: 10.1038/s41401-023-01076-9
Cell-autonomous effect of cardiomyocyte branched-chain amino acid catabolism in heart failure in mice
Abstract
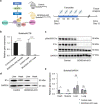
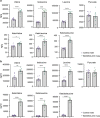
Parallel to major changes in fatty acid and glucose metabolism, defect in branched-chain amino acid (BCAA) catabolism has also been recognized as a metabolic hallmark and potential therapeutic target for heart failure. However, BCAA catabolic enzymes are ubiquitously expressed in all cell types and a systemic BCAA catabolic defect is also manifested in metabolic disorder associated with obesity and diabetes. Therefore, it remains to be determined the cell-autonomous impact of BCAA catabolic defect in cardiomyocytes in intact hearts independent from its potential global effects. In this study, we developed two mouse models. One is cardiomyocyte and temporal-specific inactivation of the E1α subunit (BCKDHA-cKO) of the branched-chain α-ketoacid dehydrogenase (BCKDH) complex, which blocks BCAA catabolism. Another model is cardiomyocyte specific inactivation of the BCKDH kinase (BCKDK-cKO), which promotes BCAA catabolism by constitutively activating BCKDH activity in adult cardiomyocytes. Functional and molecular characterizations showed E1α inactivation in cardiomyocytes was sufficient to induce loss of cardiac function, systolic chamber dilation and pathological transcriptome reprogramming. On the other hand, inactivation of BCKDK in intact heart does not have an impact on baseline cardiac function or cardiac dysfunction under pressure overload. Our results for the first time established the cardiomyocyte cell autonomous role of BCAA catabolism in cardiac physiology. These mouse lines will serve as valuable model systems to investigate the underlying mechanisms of BCAA catabolic defect induced heart failure and to provide potential insights for BCAA targeted therapy.
Keywords: branched-chain amino acid; heart failure; metabolism.
© 2023. The Author(s).
Conflict of interest statement
Rachel J Roth Flach is a Pfizer employee.
Figures