Laboratory diagnosis of Clostridioides difficile infection in symptomatic patients: what can we do better?
- PMID: 36991280
- PMCID: PMC10234961
- DOI: 10.1007/s42770-023-00956-w
Laboratory diagnosis of Clostridioides difficile infection in symptomatic patients: what can we do better?
Abstract
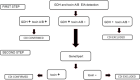
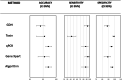
The laboratory diagnosis of Clostridioides difficile infection (CDI) is challenging since this bacteria may be detected in healthy people and toxin production detection is not sensitive enough to be used alone. Thus, there is no single test with adequate sensitivity and specificity to be used in laboratory diagnosis. We evaluated the performance of tests used in the diagnosis of CDI in symptomatic patients with risk factors in hospitals in southern Brazil. Enzyme immunoassays (EIA) for glutamate dehydrogenase antigen (GDH) and toxins A/B, real-time polymerase chain reaction (qPCR), GeneXpert system, and a two-step algorithm comprising GDH/TOXIN EIA performed simultaneously followed by GeneXpert for outliers were evaluated. Toxigenic strain in stool culture was considered CDI positive (gold standard). Among 400 samples tested, 54 (13.5%) were positive for CDI and 346 (86.5%) were negative. The diagnosis of the two-step algorithm and qPCR had an excellent performance with an accuracy of 94.5% and 94.2%, respectively. The Youden index showed that GeneXpert as a single test (83.5%) and the two-step algorithm (82.8%) were the most effective assays. Diagnosing CDI and non-CDI diarrhea could be successfully attained by the combination of clinical data with accuracy of laboratory tests.
Keywords: Antimicrobial-associated diarrhea; GDH detection; Gastrointestinal infection; Toxin A/B.
© 2023. The Author(s) under exclusive licence to Sociedade Brasileira de Microbiologia.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources