Characterization of the Response of Magnetron Sputtered In2O3-x Sensors to NO2
- PMID: 36991976
- PMCID: PMC10058676
- DOI: 10.3390/s23063265
Characterization of the Response of Magnetron Sputtered In2O3-x Sensors to NO2
Abstract
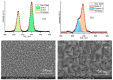
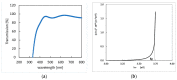
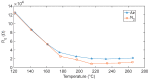
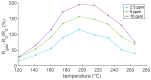
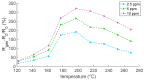
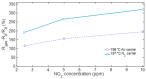
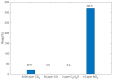
The response of resistive In2O3-x sensing devices was investigated as a function of the NO2 concentration in different operative conditions. Sensing layers are 150 nm thick films manufactured by oxygen-free room temperature magnetron sputtering deposition. This technique allows for a facile and fast manufacturing process, at same time providing advantages in terms of gas sensing performances. The oxygen deficiency during growth provides high densities of oxygen vacancies, both on the surface, where they are favoring NO2 absorption reactions, and in the bulk, where they act as donors. This n-type doping allows for conveniently lowering the thin film resistivity, thus avoiding the sophisticated electronic readout required in the case of very high resistance sensing layers. The semiconductor layer was characterized in terms of morphology, composition and electronic properties. The sensor baseline resistance is in the order of kilohms and exhibits remarkable performances with respect to gas sensitivity. The sensor response to NO2 was studied experimentally both in oxygen-rich and oxygen-free atmospheres for different NO2 concentrations and working temperatures. Experimental tests revealed a response of 32%/ppm at 10 ppm NO2 and response times of approximately 2 min at an optimal working temperature of 200 °C. The obtained performance is in line with the requirements of a realistic application scenario, such as in plant condition monitoring.
Keywords: In2O3 gas sensor; MOX gas sensor; NO2 sensors; magnetron sputtering deposition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Dhall S., Mehta B.R., Tyagi A.K., Sood K. A review on environmental gas sensors: Materials and technologies. Sens. Int. 2021;2:100116. doi: 10.1016/j.sintl.2021.100116. - DOI
-
- Raju P., Li Q. Review—Semiconductor Materials and Devices for Gas Sensors. J. Electrochem. Soc. 2022;169:057518. doi: 10.1149/1945-7111/ac6e0a. - DOI
-
- Dey A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B. 2018;229:206–217. doi: 10.1016/j.mseb.2017.12.036. - DOI
-
- Mirzaei A., Lee J.-H., Majhi S.M., Weber M., Bechelany M., Kim H.W., Kim S.S. Resistive gas sensors based on metal-oxide nanowires. J. Appl. Phys. 2019;126:241102. doi: 10.1063/1.5118805. - DOI
LinkOut - more resources
Full Text Sources

