The Debate between the Human Microbiota and Immune System in Treating Aerodigestive and Digestive Tract Cancers: A Review
- PMID: 36992076
- PMCID: PMC10055943
- DOI: 10.3390/vaccines11030492
The Debate between the Human Microbiota and Immune System in Treating Aerodigestive and Digestive Tract Cancers: A Review
Abstract
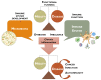
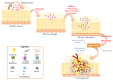
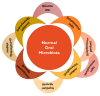
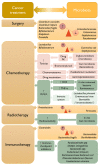
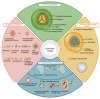
The human microbiota comprises a group of microorganisms co-existing in the human body. Unbalanced microbiota homeostasis may impact metabolic and immune system regulation, shrinking the edge between health and disease. Recently, the microbiota has been considered a prominent extrinsic/intrinsic element of cancer development and a promising milestone in the modulation of conventional cancer treatments. Particularly, the oral cavity represents a yin-and-yang target site for microorganisms that can promote human health or contribute to oral cancer development, such as Fusobacterium nucleatum. Moreover, Helicobacter pylori has also been implicated in esophageal and stomach cancers, and decreased butyrate-producing bacteria, such as Lachnospiraceae spp. and Ruminococcaceae, have demonstrated a protective role in the development of colorectal cancer. Interestingly, prebiotics, e.g., polyphenols, probiotics (Faecalibacterium, Bifidobacterium, Lactobacillus, and Burkholderia), postbiotics (inosine, butyrate, and propionate), and innovative nanomedicines can modulate antitumor immunity, circumventing resistance to conventional treatments and could complement existing therapies. Therefore, this manuscript delivers a holistic perspective on the interaction between human microbiota and cancer development and treatment, particularly in aerodigestive and digestive cancers, focusing on applying prebiotics, probiotics, and nanomedicines to overcome some challenges in treating cancer.
Keywords: cancer; cancer therapy; chemotherapy; immune system; immunotherapy; microbiome; microbiota; nanotechnology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Changes in gastric microbiota induced by Helicobacter pylori infection and preventive effects of Lactobacillus plantarum ZDY 2013 against such infection.J Dairy Sci. 2016 Feb;99(2):970-981. doi: 10.3168/jds.2015-10510. Epub 2015 Dec 17. J Dairy Sci. 2016. PMID: 26709179
-
The interplay between Helicobacter pylori and the gut microbiota: An emerging driver influencing the immune system homeostasis and gastric carcinogenesis.Front Cell Infect Microbiol. 2022 Aug 15;12:953718. doi: 10.3389/fcimb.2022.953718. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36046747 Free PMC article. Review.
-
Gut Microbiota Modulation in the Context of Immune-Related Aspects of Lactobacillus spp. and Bifidobacterium spp. in Gastrointestinal Cancers.Nutrients. 2021 Jul 31;13(8):2674. doi: 10.3390/nu13082674. Nutrients. 2021. PMID: 34444834 Free PMC article. Review.
-
In vitro modulation of human gut microbiota composition and metabolites by Bifidobacterium longum BB-46 and a citric pectin.Food Res Int. 2019 Jun;120:595-602. doi: 10.1016/j.foodres.2018.11.010. Epub 2018 Nov 9. Food Res Int. 2019. PMID: 31000276
-
Complex interactions between the microbiome and cancer immune therapy.Crit Rev Clin Lab Sci. 2019 Dec;56(8):567-585. doi: 10.1080/10408363.2019.1660303. Epub 2019 Sep 17. Crit Rev Clin Lab Sci. 2019. PMID: 31526274 Free PMC article. Review.
Cited by
-
Role of circular RNAs and gut microbiome in gastrointestinal cancers and therapeutic targets.Noncoding RNA Res. 2023 Dec 15;9(1):236-252. doi: 10.1016/j.ncrna.2023.12.002. eCollection 2024 Mar. Noncoding RNA Res. 2023. PMID: 38192436 Free PMC article. Review.
References
-
- Ribeiro A.R.P. Ph.D. Thesis. Universidade de Lisboa; Lisboa, Portugal: 2016. A Microbiota Intestinal Nas Doenças Inflamatórias do Intestino e o Potencial Recurso a Probióticos e Prebióticos.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

