Clinical and Serological Follow-Up of 216 Patients with Hematological Malignancies after Vaccination with Pfizer-BioNT162b2 mRNA COVID-19 in a Real-World Study
- PMID: 36992077
- PMCID: PMC10057553
- DOI: 10.3390/vaccines11030493
Clinical and Serological Follow-Up of 216 Patients with Hematological Malignancies after Vaccination with Pfizer-BioNT162b2 mRNA COVID-19 in a Real-World Study
Abstract
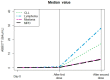
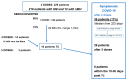
Hematological malignancies (HMs) have heterogeneous serological responses after vaccination due to disease or treatment. The aim of this real-world study was to analyze it after Pfizer-BioNT162b2 mRNA vaccination in 216 patients followed up for 1 year. The first 43 patients had an initial follow-up by a telemedicine (TM) system with no major events reported. The anti-spike IgG antibodies were checked 3-4 weeks post-first vaccination and every 3-4 months, by two standard bioassays and a rapid serological test (RST). Vaccine boosts were given when the level was <7 BAU/mL. Patients who did not seroconvert after 3-4 doses received tixagevimab/cilgavimab (TC). Fifteen results were discordant between two standard bioassays. Good agreement was observed between the standard and RST in 97 samples. After two doses, 68% were seroconverted (median = 59 BAU/mL) with a median of 162 BAU/mL and 9 BAU/mL, respectively, in untreated and treated patients (p < 0.001), particularly for patients receiving rituximab. Patients with gammaglobulin levels < 5 g/L had reduced seroconversion compared to higher levels (p = 0.019). The median levels were 228 BAU/mL post-second dose if seroconverted post-first and second, or if seroconverted only post-second dose. A total of 68% of post-second dose negative patients were post-third dose positive. A total of 16% received TC, six with non-severe symptomatic COVID-19 within 15-40 days. Personalized serological follow-up should apply particularly to patients with HMs.
Keywords: COVID-19; hematological malignancies; serological follow-up.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Real-world serological responses to extended-interval and heterologous COVID-19 mRNA vaccination in frail, older people (UNCoVER): an interim report from a prospective observational cohort study.Lancet Healthy Longev. 2022 Mar;3(3):e166-e175. doi: 10.1016/S2666-7568(22)00012-5. Epub 2022 Feb 23. Lancet Healthy Longev. 2022. PMID: 35224524 Free PMC article.
-
Quantitative Analysis of SARS-CoV-2 Antibody Levels in Cancer Patients Post Three Doses of Immunization and Prior to Breakthrough COVID-19 Infections.Curr Oncol. 2022 Sep 28;29(10):7059-7071. doi: 10.3390/curroncol29100554. Curr Oncol. 2022. PMID: 36290831 Free PMC article.
-
[Antibody Response After Two Doses of Inactivated SARS-CoV-2 Vaccine in Healthcare Workers with and without Previous COVID-19 Infection: A Prospective Observational Study].Mikrobiyol Bul. 2022 Jan;56(1):36-48. doi: 10.5578/mb.20229904. Mikrobiyol Bul. 2022. PMID: 35088958 Turkish.
-
Efficacy and safety profile of COVID-19 mRNA vaccine in patients with hematological malignancies: Systematic review and meta-analysis.Front Oncol. 2022 Aug 5;12:951215. doi: 10.3389/fonc.2022.951215. eCollection 2022. Front Oncol. 2022. PMID: 36003763 Free PMC article.
-
Third dose of anti-SARS-CoV-2 vaccine for patients with cancer: Should humoral responses be monitored? A position article.Eur J Cancer. 2022 Feb;162:182-193. doi: 10.1016/j.ejca.2021.12.011. Epub 2021 Dec 16. Eur J Cancer. 2022. PMID: 35016032 Free PMC article. Review.
Cited by
-
Azoximer bromide and hydroxyapatite: promising immune adjuvants in cancer.Cancer Biol Med. 2024 Feb 5;20(12):1021-34. doi: 10.20892/j.issn.2095-3941.2023.0222. Cancer Biol Med. 2024. PMID: 38318840 Free PMC article. Review.
References
-
- [(accessed on 17 February 2023)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19....
-
- Buske C., Dreyling M., Alvarez-Larrán A., Apperley J., Arcaini L., Besson C., Bullinger L., Corradini P., Della Porta M.G., Dimopoulos M., et al. Managing hematological cancer patients during the COVID-19 pandemic: An ESMO-EHA Interdisciplinary Expert Consensus. ESMO Open. 2022;7:100403. doi: 10.1016/j.esmoop.2022.100403. - DOI - PMC - PubMed
-
- Perry C., Luttwak E., Balaban R., Shefer G., Morales M.M., Aharon A., Tabib Y., Cohen Y.C., Benyamini N., Beyar-Katz O., et al. Efficacy of the BNT 162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv. 2021;5:3053–3061. doi: 10.1182/bloodadvances.2021005094. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

