Dynamics of Anti-S IgG Antibodies Titers after the Second Dose of COVID-19 Vaccines in the Manual and Craft Worker Population of Qatar
- PMID: 36992080
- PMCID: PMC10058349
- DOI: 10.3390/vaccines11030496
Dynamics of Anti-S IgG Antibodies Titers after the Second Dose of COVID-19 Vaccines in the Manual and Craft Worker Population of Qatar
Abstract
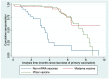
There is limited seroepidemiological evidence on the magnitude and long-term durability of antibody titers of mRNA and non-mRNA vaccines in the Qatari population. This study was conducted to generate evidence on long-term anti-S IgG antibody titers and their dynamics in individuals who have completed a primary COVID-19 vaccination schedule. A total of 300 male participants who received any of the following vaccines BNT162b2/Comirnaty, mRNA-1273, ChAdOx1-S/Covishield, COVID-19 Vaccine Janssen/Johnson, or BBIBP-CorV or Covaxin were enrolled in our study. All sera samples were tested by chemiluminescent microparticle immunoassay (CMIA) for the quantitative determination of IgG antibodies to SARS-CoV-2, receptor-binding domain (RBD) of the S1 subunit of the spike protein of SARS-CoV-2. Antibodies against SARS-CoV-2 nucleocapsid (SARS-CoV-2 N-protein IgG) were also determined. Kaplan-Meier survival curves were used to compare the time from the last dose of the primary vaccination schedule to the time by which anti-S IgG antibody titers fell into the lowest quartile (range of values collected) for the mRNA and non-mRNA vaccines. Participants vaccinated with mRNA vaccines had higher median anti-S IgG antibody titers. Participants vaccinated with the mRNA-1273 vaccine had the highest median anti-S-antibody level of 13,720.9 AU/mL (IQR 6426.5 to 30,185.6 AU/mL) followed by BNT162b2 (median, 7570.9 AU/mL; IQR, 3757.9 to 16,577.4 AU/mL); while the median anti-S antibody titer for non-mRNA vaccinated participants was 3759.7 AU/mL (IQR, 2059.7-5693.5 AU/mL). The median time to reach the lowest quartile was 3.53 months (IQR, 2.2-4.5 months) and 7.63 months (IQR, 6.3-8.4 months) for the non-mRNA vaccine recipients and Pfizer vaccine recipients, respectively. However, more than 50% of the Moderna vaccine recipients did not reach the lowest quartile by the end of the follow-up period. This evidence on anti-S IgG antibody titers should be considered for informing decisions on the durability of the neutralizing activity and thus protection against infection after the full course of primary vaccination in individuals receiving different type (mRNA verus non-mRNA) vaccines and those with natural infection.
Keywords: COVID-19; anti-S IgG; antibody titer; mRNA vaccines; non-mRNA vaccines.
Conflict of interest statement
We would like to declare that all serological test kits used in this study were provided as in-kind support for the laboratory section at Medical Commission, MOPH. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Seroepidemiological assessment of SARS-CoV-2 vaccine responsiveness and associated factors in the vaccinated community of the Casablanca-Settat Region, Morocco.Sci Rep. 2024 Apr 3;14(1):7817. doi: 10.1038/s41598-024-58498-6. Sci Rep. 2024. PMID: 38570577 Free PMC article.
-
Real-world serological responses to extended-interval and heterologous COVID-19 mRNA vaccination in frail, older people (UNCoVER): an interim report from a prospective observational cohort study.Lancet Healthy Longev. 2022 Mar;3(3):e166-e175. doi: 10.1016/S2666-7568(22)00012-5. Epub 2022 Feb 23. Lancet Healthy Longev. 2022. PMID: 35224524 Free PMC article.
-
Comparison of the effectiveness and duration of anti-RBD SARS-CoV-2 IgG antibody response between different types of vaccines: Implications for vaccine strategies.Vaccine. 2022 May 3;40(20):2841-2847. doi: 10.1016/j.vaccine.2022.03.069. Epub 2022 Apr 1. Vaccine. 2022. PMID: 35397946 Free PMC article.
-
Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study.Lancet Haematol. 2021 Aug;8(8):e583-e592. doi: 10.1016/S2352-3026(21)00169-1. Epub 2021 Jul 2. Lancet Haematol. 2021. PMID: 34224668 Free PMC article.
-
Overview of the Main Anti-SARS-CoV-2 Vaccines: Mechanism of Action, Efficacy and Safety.Infect Drug Resist. 2021 Aug 31;14:3459-3476. doi: 10.2147/IDR.S315727. eCollection 2021. Infect Drug Resist. 2021. PMID: 34511939 Free PMC article. Review.
Cited by
-
Effectiveness and duration of additional immune defense provided by SARS-CoV-2 infection before and after receiving the mRNA COVID-19 vaccine BNT162b2.Vaccine X. 2024 Jun 26;19:100518. doi: 10.1016/j.jvacx.2024.100518. eCollection 2024 Aug. Vaccine X. 2024. PMID: 39040888 Free PMC article.
-
Anti-S and Anti-N Antibody Responses of COVID-19 Vaccine Recipients.Vaccines (Basel). 2023 Aug 22;11(9):1398. doi: 10.3390/vaccines11091398. Vaccines (Basel). 2023. PMID: 37766076 Free PMC article.
-
The Antibodies' Response to SARS-CoV-2 Vaccination: 1-Year Follow Up.Biomedicines. 2023 Sep 28;11(10):2661. doi: 10.3390/biomedicines11102661. Biomedicines. 2023. PMID: 37893035 Free PMC article.
References
-
- WHO WHO Coronavirus (COVID-19) Dashboard. 2022. [(accessed on 5 September 2022)]. Available online: https://covid19.who.int/?gclid=EAIaIQobChMIkazL-ojJ8gIVjpGyCh1iEgKXEAAYA....
-
- MOPH Ministry of Public Health Qatar 2022. [(accessed on 5 September 2022)]; Available online: https://covid19.moph.gov.qa/EN/Pages/default.aspx.
-
- Hall V.J., Foulkes S., Charlett A., Atti A., Monk E.J.M., Simmons R., Wellington E., Cole M.J., Saei A., Oguti B., et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: A large, multicentre, prospective cohort study (SIREN) Lancet. 2021;397:1459–1469. doi: 10.1016/S0140-6736(21)00675-9. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

