mRNA-Based Vaccine for COVID-19: They Are New but Not Unknown!
- PMID: 36992091
- PMCID: PMC10052021
- DOI: 10.3390/vaccines11030507
mRNA-Based Vaccine for COVID-19: They Are New but Not Unknown!
Abstract
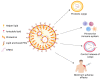
mRNA vaccines take advantage of the mechanism that our cells use to produce proteins. Our cells produce proteins based on the knowledge contained in our DNA; each gene encodes a unique protein. The genetic information is essential, but cells cannot use it until mRNA molecules convert it into instructions for producing specific proteins. mRNA vaccinations provide ready-to-use mRNA instructions for constructing a specific protein. BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) both are newly approved mRNA-based COVID-19 vaccines that have shown excellent protection and efficacy. In total, there are five more mRNA-based vaccine candidates for COVID-19 under different phases of clinical development. This review is specifically focused on mRNA-based vaccines for COVID-19 covering its development, mechanism, and clinical aspects.
Keywords: COVID-19 vaccine; mRNA vaccines; vaccine development; vector-based vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- WHO WHO Coronavirus (COVID-19) Dashboard 2023. [(accessed on 17 January 2023)]; Available online: https://covid19.who.int/
Publication types
LinkOut - more resources
Full Text Sources

