Is SARS-CoV-2 Vaccination of Subjects with a Prior History of Allergies Dangerous? Experiences in the Veneto Region of Italy
- PMID: 36992158
- PMCID: PMC10052958
- DOI: 10.3390/vaccines11030574
Is SARS-CoV-2 Vaccination of Subjects with a Prior History of Allergies Dangerous? Experiences in the Veneto Region of Italy
Abstract
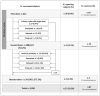
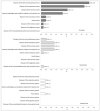
Adverse events after SARS-CoV-2 vaccinations have caused alarm to some individuals with previously diagnosed allergies. The aim of this study was to investigate whether the risk of adverse reactions was actually higher in this subgroup. To this end, we carried out an observational descriptive analysis of vaccines administered in a "protected setting" in the Veneto region of Italy between December 2020 and December 2022. Reactions were classified using systemic organic classification (SOC), and their severity was assessed using the criteria of the Italian Drug Agency (AIFA). A total of 421 subjects were vaccinated with 1050 doses, 95.0% of which were administered without adverse events. In all, 53 subjects reported 87 SOC reactions (1.6 reactions/person), and 18.3% of these reactions were severe. One person was hospitalized, but all subjects enjoyed complete remission. Reporting rates were 9.0%, 3.1%, and 1.2% for first, second, and third doses, respectively. The most frequent reactions involved the respiratory system (2.3%), the cutaneous and subcutaneous systems (2.1%), and the nervous system (1.7%). Multivariate analyses (adjOR (95% CI)) revealed that the probability of experiencing at least one reaction significantly declined with increases in age [0.95 (0.94-0.97)] and in the number of doses received, i.e., 75% [0.25 (0.13-0.49)] for second doses and 88% [0.12 (0.04-0.39)] for third doses. These results indicated that vaccinations could be safely administered; few reactions were reported, and there were no permanent adverse outcomes.
Keywords: SARS-CoV-2; allergies; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

