BMI-Associated Anti-Apolipoprotein A-1 Positivity in Healthy Adults after mRNA-Vaccination against COVID-19
- PMID: 36992254
- PMCID: PMC10053701
- DOI: 10.3390/vaccines11030670
BMI-Associated Anti-Apolipoprotein A-1 Positivity in Healthy Adults after mRNA-Vaccination against COVID-19
Abstract
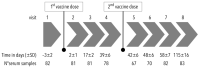
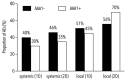
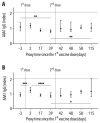
Elevated anti-apolipoprotein A-1 (AAA1) antibody levels associated with cardiovascular risk have been observed in previously SARS-CoV-2-infected or COVID-19-vaccinated individuals. Since patient safety is generally a priority in vaccination, we sought to investigate AAA1 antibody levels in healthy adults after mRNA vaccination. We conducted a prospective cohort study in healthy adult volunteers recruited from military workers of the Transport Air Base in Prague who had received two doses of mRNA vaccines. Anti-apolipoprotein A-1 antibody levels were determined using ELISA from serum samples obtained at three and four time points after the first and second vaccine doses, respectively, within almost 17 weeks of follow-up. The transient AAA1 positivity rate achieved 24.1% (95% confidence interval CI: 15.4-34.7%), i.e., 20 out of 83 participants had at least one positive post-vaccination sample, with a repeat positivity confirmed in only 5 of them. This rate was associated with a BMI > 26 kg/m2, as documented by an adjusted odds ratio of 6.79 (95% CI: 1.53-30.01). In addition, the highest positivity rate of 46.7% (21.3-73.4%) was observed in obese subjects with >30 kg/m2. Since the incidence rate of AAA1 positivity remained unchanged after the first and second vaccine doses, any relationship between AAA1 positivity and mRNA vaccination was inconclusive. The present study showed a transient AAA1 positivity rate associated with overweight or obesity without a proven association with mRNA vaccination.
Keywords: BMI; anti-apolipoprotein A-1; autoantibody; mRNA vaccination; obesity.
Conflict of interest statement
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; and in the decision to publish the results.
Figures
Similar articles
-
Anti-SARS-CoV-2 mRNA vaccines as inducers of humoral response against apolipoprotein A-1?Eur J Clin Invest. 2022 Feb;52(2):e13713. doi: 10.1111/eci.13713. Epub 2021 Nov 29. Eur J Clin Invest. 2022. PMID: 34841527 Free PMC article.
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Real-world serological responses to extended-interval and heterologous COVID-19 mRNA vaccination in frail, older people (UNCoVER): an interim report from a prospective observational cohort study.Lancet Healthy Longev. 2022 Mar;3(3):e166-e175. doi: 10.1016/S2666-7568(22)00012-5. Epub 2022 Feb 23. Lancet Healthy Longev. 2022. PMID: 35224524 Free PMC article.
-
Healthcare Workers in South Korea Maintain a SARS-CoV-2 Antibody Response Six Months After Receiving a Second Dose of the BNT162b2 mRNA Vaccine.Front Immunol. 2022 Jan 31;13:827306. doi: 10.3389/fimmu.2022.827306. eCollection 2022. Front Immunol. 2022. PMID: 35173736 Free PMC article.
-
[Antibody Response After Two Doses of Inactivated SARS-CoV-2 Vaccine in Healthcare Workers with and without Previous COVID-19 Infection: A Prospective Observational Study].Mikrobiyol Bul. 2022 Jan;56(1):36-48. doi: 10.5578/mb.20229904. Mikrobiyol Bul. 2022. PMID: 35088958 Turkish.
Cited by
-
Evaluation of serum and urine biomarkers for severe COVID-19.Front Med (Lausanne). 2024 Mar 6;11:1357659. doi: 10.3389/fmed.2024.1357659. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38510452 Free PMC article.
References
-
- Munro A.P.S., Janani L., Cornelius V., Aley P.K., Babbage G., Baxter D., Bula M., Cathie K., Chatterjee K., Dodd K., et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 NCoV-19 or BNT162b2 in the UK (CoV-BOOST): A blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398:2258–2276. doi: 10.1016/S0140-6736(21)02717-3. - DOI - PMC - PubMed
-
- Stuart A.S.V., Shaw R.H., Liu X., Greenland M., Aley P.K., Andrews N.J., Cameron J.C., Charlton S., Clutterbuck E.A., Collins A.M., et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating MRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-CoV2): A single-blind, randomised, phase 2, non-inferiority trial. Lancet. 2022;399:36–49. doi: 10.1016/S0140-6736(21)02718-5. - DOI - PMC - PubMed
-
- Adjobimey T., Meyer J., Sollberg L., Bawolt M., Berens C., Kovačević P., Trudić A., Parcina M., Hoerauf A. Comparison of IgA, IgG, and neutralizing antibody responses following immunization with moderna, biontech, AstraZeneca, Sputnik-V, Johnson and Johnson, and Sinopharm’s COVID-19 vaccines. Front. Immunol. 2022;13:917905. doi: 10.3389/fimmu.2022.917905. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

