SARS-CoV-2 Vaccines, Vaccine Development Technologies, and Significant Efforts in Vaccine Development during the Pandemic: The Lessons Learned Might Help to Fight against the Next Pandemic
- PMID: 36992266
- PMCID: PMC10054865
- DOI: 10.3390/vaccines11030682
SARS-CoV-2 Vaccines, Vaccine Development Technologies, and Significant Efforts in Vaccine Development during the Pandemic: The Lessons Learned Might Help to Fight against the Next Pandemic
Abstract
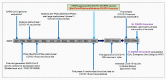
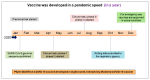
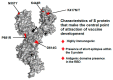
We are currently approaching three years since the beginning of the coronavirus disease 2019 (COVID-19) pandemic. SARS-CoV-2 has caused extensive disruptions in everyday life, public health, and the global economy. Thus far, the vaccine has worked better than expected against the virus. During the pandemic, we experienced several things, such as the virus and its pathogenesis, clinical manifestations, and treatments; emerging variants; different vaccines; and the vaccine development processes. This review describes how each vaccine has been developed and approved with the help of modern technology. We also discuss critical milestones during the vaccine development process. Several lessons were learned from different countries during the two years of vaccine research, development, clinical trials, and vaccination. The lessons learned during the vaccine development process will help to fight the next pandemic.
Keywords: COVID-19 pandemic; SARS-CoV-2; lesson learned; vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- da Silva S.J.R., do Nascimento J.C.F., Germano Mendes R.P., Guarines K.M., da Silva C.T.A., da Silva P.G., de Magalhaes J.J.F., Vigar J.R.J., Silva-Junior A., Kohl A., et al. Two Years into the COVID-19 Pandemic: Lessons Learned. ACS Infect. Dis. 2022;8:1758–1814. doi: 10.1021/acsinfecdis.2c00204. - DOI - PMC - PubMed
-
- Kumar R., Rai A.K., Phukan M.M., Hussain A., Borah D., Gogoi B., Chakraborty P., Buragohain A.K. Accumulating Impact of Smoking and Co-morbidities on Severity and Mortality of COVID-19 Infection: A Systematic Review and Meta-analysis. Curr. Genom. 2021;22:339–352. doi: 10.2174/1389202922666210921101728. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

