Impact of Previous Common Human Coronavirus Exposure on SARS-CoV-2-Specific T-Cell and Memory B-Cell Response after mRNA-Based Vaccination
- PMID: 36992336
- PMCID: PMC10059801
- DOI: 10.3390/v15030627
Impact of Previous Common Human Coronavirus Exposure on SARS-CoV-2-Specific T-Cell and Memory B-Cell Response after mRNA-Based Vaccination
Abstract
Objective: T-cell responses against SARS-CoV-2 are observed in unexposed individuals, attributed to previous common human coronavirus (HCoV) infections. We evaluated the evolution of this T-cell cross-reactive response and the specific memory B-cells (MBCs) after the SARS-CoV-2 mRNA-based vaccination and its impact on incident SARS-CoV-2 infections.
Methods: This was a longitudinal study of 149 healthcare workers (HCWs) that included 85 unexposed individuals that were subdivided according to previous T-cell cross-reactivity, who were compared to 64 convalescent HCWs. Changes in specific T-cell response and memory B-cell (MBC) levels were compared at baseline and after two doses of the SARS-CoV-2 mRNA-based vaccine.
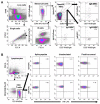
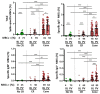
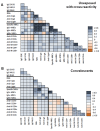
Results: A cross-reactive T-cell response was found in 59% of unexposed individuals before vaccination. Antibodies against HKU1 positively correlated with OC43 and 229E antibodies. Spike-specific MBCs was scarce in unexposed HCWs regardless of the presence of baseline T-cell cross-reactivity. After vaccination, 92% and 96% of unexposed HCWs with cross-reactive T-cells had CD4+ and CD8+ T-cell responses to the spike protein, respectively. Similar results to that were found in convalescents (83% and 92%, respectively). Contrarily, higher than that which was observed in unexposed individuals without T-cell cross-reactivity showed lower CD4+ and CD8+ T-cell responses (73% in both cases, p = 0.03). Nevertheless, previous cross-reactive T-cell response was not associated with higher levels of MBCs after vaccination in unexposed HCWs. During a follow-up of 434 days (IQR, 339-495) after vaccination, 49 HCWs (33%) became infected, with a significant positive correlation between spike-specific MBC levels and isotypes IgG+ and IgA+ after vaccination and a longer time to get infected. Interestingly, T-cell cross-reactivity did not reduce the time to vaccine breakthrough infections.
Conclusion: While pre-existing T-cell cross-reactivity enhances the T-cell response after vaccination, it does not increase SARS-CoV-2-specific MBC levels in the absence of previous infection. Overall, the level of specific MBCs determines the time to breakthrough infections, regardless of the presence of T-cell cross-reactivity.
Keywords: SARS-CoV-2; coronavirus; cross-reactive; mRNA vaccine; memory B-cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Goel R.R., Apostolidis S.A., Painter M.M., Mathew D., Pattekar A., Kuthuru O., Gouma S., Hicks P., Meng W., Rosenfeld A.M., et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naive and recovered individuals following mRNA vaccination. Sci. Immunol. 2021;6:eabi6950. doi: 10.1126/sciimmunol.abi6950. - DOI - PMC - PubMed
-
- Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S., et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181:1489–1501.E15. doi: 10.1016/j.cell.2020.05.015. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

