Advances in Antiviral Delivery Systems and Chitosan-Based Polymeric and Nanoparticulate Antivirals and Antiviral Carriers
- PMID: 36992356
- PMCID: PMC10054433
- DOI: 10.3390/v15030647
Advances in Antiviral Delivery Systems and Chitosan-Based Polymeric and Nanoparticulate Antivirals and Antiviral Carriers
Abstract
Current antiviral therapy research is focused on developing dosage forms that enable highly effective drug delivery, providing a selective effect in the organism, lower risk of adverse effects, a lower dose of active pharmaceutical ingredients, and minimal toxicity. In this article, antiviral drugs and the mechanisms of their action are summarized at the beginning as a prerequisite background to develop relevant drug delivery/carrier systems for them, classified and briefly discussed subsequently. Many of the recent studies aim at different types of synthetic, semisynthetic, and natural polymers serving as a favorable matrix for the antiviral drug carrier. Besides a wider view of different antiviral delivery systems, this review focuses on advances in antiviral drug delivery systems based on chitosan (CS) and derivatized CS carriers. CS and its derivatives are evaluated concerning methods of their preparation, their basic characteristics and properties, approaches to the incorporation of an antiviral drug in the CS polymer as well as CS nanoparticulate systems, and their recent biomedical applications in the context of actual antiviral therapy. The degree of development (i.e., research study, in vitro/ex vivo/in vivo preclinical testing), as well as benefits and limitations of CS polymer and CS nanoparticulate drug delivery systems, are reported for particular viral diseases and corresponding antivirotics.
Keywords: antivirotic; chitosan; chitosan derivatives; chitosan nanocomposites; drug delivery; nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
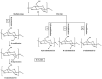

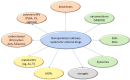

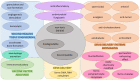
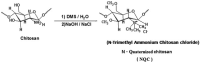




Similar articles
-
Advances in Chitosan-Based Nanoparticles for Drug Delivery.Int J Mol Sci. 2021 Sep 6;22(17):9652. doi: 10.3390/ijms22179652. Int J Mol Sci. 2021. PMID: 34502560 Free PMC article. Review.
-
Recent advances in chitosan-based nanoparticulate pulmonary drug delivery.Nanoscale. 2016 Aug 14;8(30):14341-58. doi: 10.1039/c6nr03256g. Epub 2016 Jul 20. Nanoscale. 2016. PMID: 27439116 Review.
-
Polymeric nanoencapsulation of alpha interferon increases drug bioavailability and induces a sustained antiviral response in vivo.Mater Sci Eng C Mater Biol Appl. 2020 Nov;116:111260. doi: 10.1016/j.msec.2020.111260. Epub 2020 Jul 6. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 32806331
-
Advances in the delivery of anticancer drugs by nanoparticles and chitosan-based nanoparticles.Int J Pharm X. 2024 Aug 28;8:100281. doi: 10.1016/j.ijpx.2024.100281. eCollection 2024 Dec. Int J Pharm X. 2024. PMID: 39297017 Free PMC article. Review.
-
Chitosan Derivatives as Carriers for Drug Delivery and Biomedical Applications.ACS Biomater Sci Eng. 2023 May 8;9(5):2181-2202. doi: 10.1021/acsbiomaterials.2c01297. Epub 2023 Apr 10. ACS Biomater Sci Eng. 2023. PMID: 37036371 Review.
Cited by
-
Exploring the role of density functional theory in the design of gold nanoparticles for targeted drug delivery: a systematic review.J Mol Model. 2025 Jun 10;31(7):186. doi: 10.1007/s00894-025-06405-9. J Mol Model. 2025. PMID: 40493107 Review.
-
Polydopamine-based nanomedicines for efficient antiviral and secondary injury protection therapy.Sci Adv. 2023 Jun 16;9(24):eadf4098. doi: 10.1126/sciadv.adf4098. Epub 2023 Jun 14. Sci Adv. 2023. PMID: 37315148 Free PMC article.
-
Chitosan in Oral Drug Delivery Formulations: A Review.Pharmaceutics. 2023 Sep 21;15(9):2361. doi: 10.3390/pharmaceutics15092361. Pharmaceutics. 2023. PMID: 37765329 Free PMC article. Review.
-
Strategies and efforts in circumventing the emergence of antiviral resistance against conventional antivirals.NPJ Antimicrob Resist. 2025 Jun 9;3(1):54. doi: 10.1038/s44259-025-00125-z. NPJ Antimicrob Resist. 2025. PMID: 40490516 Free PMC article. Review.
-
Antiviral nanomedicine: Advantages, mechanisms and advanced therapies.Bioact Mater. 2025 Jun 5;52:92-122. doi: 10.1016/j.bioactmat.2025.05.030. eCollection 2025 Oct. Bioact Mater. 2025. PMID: 40530413 Free PMC article. Review.
References
-
- Saxena S.K., Mishra N., Saxena R. Advances in antiviral drug discovery and development: Part I: Advancements in antiviral drug discovery. Future Virol. 2009;4:101–107. doi: 10.2217/17460794.4.2.101. - DOI
-
- De Clercq E. Chemotherapy of Viral Infections. In: Baron S., editor. Medical Microbiology. 4th ed. University of Texas Medical Branch at Galveston; Galveston, TX, USA: 1996. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

