Serum Lactate Dehydrogenase Level One Week after Admission Is the Strongest Predictor of Prognosis of COVID-19: A Large Observational Study Using the COVID-19 Registry Japan
- PMID: 36992380
- PMCID: PMC10058713
- DOI: 10.3390/v15030671
Serum Lactate Dehydrogenase Level One Week after Admission Is the Strongest Predictor of Prognosis of COVID-19: A Large Observational Study Using the COVID-19 Registry Japan
Abstract
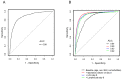
Clinical features of COVID-19 are diverse, and a useful tool for predicting clinical outcomes based on clinical characteristics of COVID-19 is needed. This study examined the laboratory values and trends that influence mortality in hospitalised COVID-19 patients. Data on hospitalised patients enrolled in a registry study in Japan (COVID-19 Registry Japan) were obtained. Patients with records on basic information, outcomes, and laboratory data on the day of admission (day 1) and day 8 were included. In-hospital mortality was set as the outcome, and associated factors were identified by multivariate analysis using the stepwise method. A total of 8860 hospitalised patients were included. The group with lactate dehydrogenase (LDH) levels >222 IU/L on day 8 had a higher mortality rate compared to the group with LDH levels ≤222 IU/L. Similar results were observed in subgroups formed by age, body mass index (BMI), underlying disease, and mutation type, except for those aged <50 years. When age, sex, BMI, underlying disease, and laboratory values on days 1 and 8 were tested for factors strongly associated with in-hospital mortality, LDH on day 8 was most strongly associated with mortality. LDH level on day 8 was the strongest predictor of in-hospital mortality in hospitalised COVID-19 patients, indicating its potential usefulness in post-treatment decision-making in severe COVID-19 cases.
Keywords: COVID-19; COVID-19 registry Japan; SARS-CoV-2; lactate dehydrogenase.
Conflict of interest statement
IY reports grants from KAKENHI, AMED, and Health, Labour and Welfare Policy Research Grants, research fund by Nihon Medi-Physics, and speaker fees from Chugai Pharmaceutical Co and AstraZeneca outside the submitted work. The other authors declare that they have no conflicts of interest.
Figures


References
-
- WHO COVID-19 Dashboard Geneva: World Health Organization. [(accessed on 15 February 2023)]. Available online: https://covid19.who.int/
-
- Lopez Bernal J., Andrews N., Gower C., Robertson C., Stowe J., Tessier E., Simmons R., Cottrell S., Roberts R., O’Doherty M., et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. - DOI - PMC - PubMed
-
- Wong C.K.H., Au I.C.H., Lau K.T.K., Lau E.H.Y., Cowling B.J., Leung G.M. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong’s omicron BA.2 wave: A retrospective cohort study. Lancet Infect. Dis. 2022;22:1681–1693. doi: 10.1016/S1473-3099(22)00507-2. - DOI - PMC - PubMed
-
- Bager P., Wohlfahrt J., Bhatt S., Stegger M., Legarth R., Møller C.H., Skov R.L., Valentiner-Branth P., Voldstedlund M., Fischer T.K., et al. Risk of hospitalisation associated with infection with SARS-CoV-2 omicron variant versus delta variant in Denmark: An observational cohort study. Lancet Infect. Dis. 2022;22:967–976. doi: 10.1016/S1473-3099(22)00154-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

