Cellular Targets of HIV-1 Protease: Just the Tip of the Iceberg?
- PMID: 36992421
- PMCID: PMC10053624
- DOI: 10.3390/v15030712
Cellular Targets of HIV-1 Protease: Just the Tip of the Iceberg?
Abstract
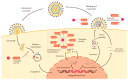
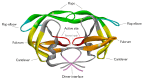
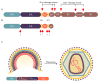
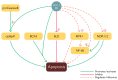
Human immunodeficiency virus 1 (HIV-1) viral protease (PR) is one of the most studied viral enzymes and a crucial antiviral target. Despite its well-characterized role in virion maturation, an increasing body of research is starting to focus on its ability to cleave host cell proteins. Such findings are apparently in contrast with the dogma of HIV-1 PR activity being restricted to the interior of nascent virions and suggest catalytic activity within the host cell environment. Given the limited amount of PR present in the virion at the time of infection, such events mainly occur during late viral gene expression, mediated by newly synthesized Gag-Pol polyprotein precursors, rather than before proviral integration. HIV-1 PR mainly targets proteins involved in three different processes: those involved in translation, those controlling cell survival, and restriction factors responsible for innate/intrinsic antiviral responses. Indeed, by cleaving host cell translation initiation factors, HIV-1 PR can impair cap-dependent translation, thus promoting IRES-mediated translation of late viral transcripts and viral production. By targeting several apoptotic factors, it modulates cell survival, thus promoting immune evasion and viral dissemination. Additionally, HIV-1 PR counteracts restriction factors incorporated in the virion that would otherwise interfere with nascent virus vitality. Thus, HIV-1 PR appears to modulate host cell function at different times and locations during its life cycle, thereby ensuring efficient viral persistency and propagation. However, we are far from having a complete picture of PR-mediated host cell modulation, which is emerging as a field that needs further investigation.
Keywords: HIV-1 PR; antiviral therapy; apoptosis; cell death; host cell shut-off; host factors; protease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- IN DANGER: UNAIDS Global AIDS Update 2022. Joint United Nations Programme on HIV/AIDS; Geneva, Switzerland: 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

