Manipulation of the Cellular Membrane-Cytoskeleton Network for RNA Virus Replication and Movement in Plants
- PMID: 36992453
- PMCID: PMC10056259
- DOI: 10.3390/v15030744
Manipulation of the Cellular Membrane-Cytoskeleton Network for RNA Virus Replication and Movement in Plants
Abstract
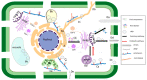
Viruses infect all cellular life forms and cause various diseases and significant economic losses worldwide. The majority of viruses are positive-sense RNA viruses. A common feature of infection by diverse RNA viruses is to induce the formation of altered membrane structures in infected host cells. Indeed, upon entry into host cells, plant-infecting RNA viruses target preferred organelles of the cellular endomembrane system and remodel organellar membranes to form organelle-like structures for virus genome replication, termed as the viral replication organelle (VRO) or the viral replication complex (VRC). Different viruses may recruit different host factors for membrane modifications. These membrane-enclosed virus-induced replication factories provide an optimum, protective microenvironment to concentrate viral and host components for robust viral replication. Although different viruses prefer specific organelles to build VROs, at least some of them have the ability to exploit alternative organellar membranes for replication. Besides being responsible for viral replication, VROs of some viruses can be mobile to reach plasmodesmata (PD) via the endomembrane system, as well as the cytoskeleton machinery. Viral movement protein (MP) and/or MP-associated viral movement complexes also exploit the endomembrane-cytoskeleton network for trafficking to PD where progeny viruses pass through the cell-wall barrier to enter neighboring cells.
Keywords: cytoskeleton; endomembrane; plant virus; positive-sense RNA virus; virus intercellular movement; virus replication; virus-plant interaction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

