Elucidating the Implications of Norovirus N- and O-Glycosylation, O-GlcNAcylation, and Phosphorylation
- PMID: 36992506
- PMCID: PMC10054809
- DOI: 10.3390/v15030798
Elucidating the Implications of Norovirus N- and O-Glycosylation, O-GlcNAcylation, and Phosphorylation
Abstract
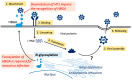
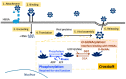
Norovirus is the most common cause of foodborne gastroenteritis, affecting millions of people worldwide annually. Among the ten genotypes (GI-GX) of norovirus, only GI, GII, GIV, GVIII, and GIX infect humans. Some genotypes reportedly exhibit post-translational modifications (PTMs), including N- and O-glycosylation, O-GlcNAcylation, and phosphorylation, in their viral antigens. PTMs have been linked to increased viral genome replication, viral particle release, and virulence. Owing to breakthroughs in mass spectrometry (MS) technologies, more PTMs have been discovered in recent years and have contributed significantly to preventing and treating infectious diseases. However, the mechanisms by which PTMs act on noroviruses remain poorly understood. In this section, we outline the current knowledge of the three common types of PTM and investigate their impact on norovirus pathogenesis. Moreover, we summarize the strategies and techniques for the identification of PTMs.
Keywords: N- and O-glycosylation; O-GlcNAcylation; norovirus; phosphorylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Pires S.M., Fischer-Walker C.L., Lanata C.F., Devleesschauwer B., Hall A.J., Kirk M.D., Duarte A.S., Black R.E., Angulo F.J. Aetiology-Specific Estimates of the Global and Regional Incidence and Mortality of Diarrhoeal Diseases Commonly Transmitted through Food. PLoS ONE. 2015;10:e0142927. doi: 10.1371/journal.pone.0142927. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

