This is a preprint.
Refining the serum miR-371a-3p test for viable germ cell tumor detection: identification and definition of an indeterminate range
- PMID: 36993198
- PMCID: PMC10055551
- DOI: 10.21203/rs.3.rs-2644890/v1
Refining the serum miR-371a-3p test for viable germ cell tumor detection: identification and definition of an indeterminate range
Update in
-
Refining the serum miR-371a-3p test for viable germ cell tumor detection.Sci Rep. 2023 Jun 29;13(1):10558. doi: 10.1038/s41598-023-37271-1. Sci Rep. 2023. PMID: 37386046 Free PMC article.
Abstract
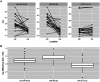
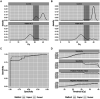
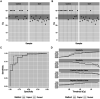
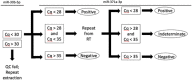
Circulating miR-371a-3p has excellent performance in the detection of viable (non-teratoma) GCT pre-orchiectomy; however, its ability to detect occult disease is understudied. To refine the serum miR-371a-3p assay in the minimal residual disease setting we compared performance of raw (Cq) and normalized (∆Cq, RQ) values from prior assays, and validated interlaboratory concordance by aliquot swapping. Revised assay performance was determined in a cohort of 32 patients suspected of occult retroperitoneal disease. Assay superiority was determined by comparing resulting receiver-operator characteristic (ROC) curves using the Delong method. Pairwise t-tests were used to test for interlaboratory concordance. Performance was comparable when thresholding based on raw Cq vs. normalized values. Interlaboratory concordance of miR-371a-3p was high, but reference genes miR-30b-5p and cel-miR-39-3p were discordant. Introduction of an indeterminate range of Cq 28-35 with a repeat run for any indeterminate improved assay accuracy from 0.84 to 0.92 in a group of patients suspected of occult GCT. We recommend that serum miR-371a-3p test protocols are updated to a) utilize threshold-based approaches using raw Cq values, b) continue to include an endogenous (e.g., miR-30b-5p) and exogenous non-human spike-in (e.g., cel-miR-39-3p) microRNA for quality control, and c) to re-run any sample with an indeterminate result.
Figures
References
-
- Saoud R. M., Andolfi C., Aizen J. et al.: Impact of Non-guideline-directed Care on Quality of Life in Testicular Cancer Survivors. Eur Urol Focus, 7: 1137, 2021 - PubMed
-
- Wymer K. M., Daneshmand S., Pierorazio P. M. et al.: Mildly elevated serum alpha-fetoprotein (AFP) among patients with testicular cancer may not be associated with residual cancer or need for treatment. Ann Oncol, 28: 899, 2017 - PubMed
-
- Ehrlich Y., Brames M. J., Beck S. D. et al.: Long-term follow-up of Cisplatin combination chemotherapy in patients with disseminated nonseminomatous germ cell tumors: is a postchemotherapy retroperitoneal lymph node dissection needed after complete remission? J Clin Oncol, 28: 531, 2010 - PubMed
-
- Chakiryan N. H., Dahmen A., Cucchiara V. et al.: Reliability of Serum Tumor Marker Measurement to Diagnose Recurrence in Patients with Clinical Stage I Nonseminomatous Germ Cell Tumors Undergoing Active Surveillance: A Systematic Review. J Urol, 205: 1569, 2021 - PubMed
-
- Kollmannsberger C., Tandstad T., Bedard P. L. et al.: Patterns of relapse in patients with clinical stage I testicular cancer managed with active surveillance. J Clin Oncol, 33: 51, 2015 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

