This is a preprint.
Bipartite invariance in mouse primary visual cortex
- PMID: 36993218
- PMCID: PMC10055119
- DOI: 10.1101/2023.03.15.532836
Bipartite invariance in mouse primary visual cortex
Abstract
A defining characteristic of intelligent systems, whether natural or artificial, is the ability to generalize and infer behaviorally relevant latent causes from high-dimensional sensory input, despite significant variations in the environment. To understand how brains achieve generalization, it is crucial to identify the features to which neurons respond selectively and invariantly. However, the high-dimensional nature of visual inputs, the non-linearity of information processing in the brain, and limited experimental time make it challenging to systematically characterize neuronal tuning and invariances, especially for natural stimuli. Here, we extended "inception loops" - a paradigm that iterates between large-scale recordings, neural predictive models, and in silico experiments followed by in vivo verification - to systematically characterize single neuron invariances in the mouse primary visual cortex. Using the predictive model we synthesized Diverse Exciting Inputs (DEIs), a set of inputs that differ substantially from each other while each driving a target neuron strongly, and verified these DEIs' efficacy in vivo. We discovered a novel bipartite invariance: one portion of the receptive field encoded phase-invariant texture-like patterns, while the other portion encoded a fixed spatial pattern. Our analysis revealed that the division between the fixed and invariant portions of the receptive fields aligns with object boundaries defined by spatial frequency differences present in highly activating natural images. These findings suggest that bipartite invariance might play a role in segmentation by detecting texture-defined object boundaries, independent of the phase of the texture. We also replicated these bipartite DEIs in the functional connectomics MICrONs data set, which opens the way towards a circuit-level mechanistic understanding of this novel type of invariance. Our study demonstrates the power of using a data-driven deep learning approach to systematically characterize neuronal invariances. By applying this method across the visual hierarchy, cell types, and sensory modalities, we can decipher how latent variables are robustly extracted from natural scenes, leading to a deeper understanding of generalization.
Figures

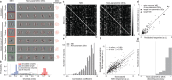

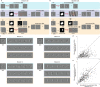

References
-
- Gross Charles G, de Rocha-Miranda CE, and Bender DB. Visual properties of neurons in inferotemporal cortex of the macaque. Journal of neurophysiology, 35(1):96–111, 1972. - PubMed
-
- Yamins Daniel LK and DiCarlo James J. Using goal-driven deep learning models to understand sensory cortex. Nature neuroscience, 19(3):356–365, 2016. - PubMed
-
- Cadieu Charles, Kouh Minjoon, Pasupathy Anitha, Connor Charles E, Riesenhuber Maximilian, and Poggio Tomaso. A model of v4 shape selectivity and invariance. Journal of neurophysiology, 98(3):1733–1750, 2007. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
