This is a preprint.
Aversive experience drives offline ensemble reactivation to link memories across days
- PMID: 36993254
- PMCID: PMC10054942
- DOI: 10.1101/2023.03.13.532469
Aversive experience drives offline ensemble reactivation to link memories across days
Update in
-
Offline ensemble co-reactivation links memories across days.Nature. 2025 Jan;637(8044):145-155. doi: 10.1038/s41586-024-08168-4. Epub 2024 Nov 6. Nature. 2025. PMID: 39506117 Free PMC article.
Abstract
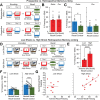
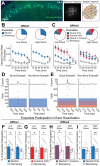
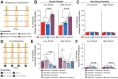
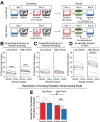
Memories are encoded in neural ensembles during learning and stabilized by post-learning reactivation. Integrating recent experiences into existing memories ensures that memories contain the most recently available information, but how the brain accomplishes this critical process remains unknown. Here we show that in mice, a strong aversive experience drives the offline ensemble reactivation of not only the recent aversive memory but also a neutral memory formed two days prior, linking the fear from the recent aversive memory to the previous neutral memory. We find that fear specifically links retrospectively, but not prospectively, to neutral memories across days. Consistent with prior studies, we find reactivation of the recent aversive memory ensemble during the offline period following learning. However, a strong aversive experience also increases co-reactivation of the aversive and neutral memory ensembles during the offline period. Finally, the expression of fear in the neutral context is associated with reactivation of the shared ensemble between the aversive and neutral memories. Taken together, these results demonstrate that strong aversive experience can drive retrospective memory-linking through the offline co-reactivation of recent memory ensembles with memory ensembles formed days prior, providing a neural mechanism by which memories can be integrated across days.
Keywords: PTSD; co-firing; ensemble; hippocampus; memory integration; memory-linking; offline periods; reactivation; stress.
Conflict of interest statement
Competing Interests The authors declare no competing interests.
Figures





References
-
- Reijmers L. G., Perkins B. L., Matsuo N. & Mayford M. Localization of a stable neural correlate of associative memory. Science 317, 1230–1233 (2007). https://doi.org:10.1126/science.1143839 - DOI - PubMed
-
- Liu X. et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012). https://doi.org:10.1038/nature11028 - DOI - PMC - PubMed
-
- Han J. H. et al. Neuronal competition and selection during memory formation. Science 316, 457–460 (2007). https://doi.org:10.1126/science.1139438 - DOI - PubMed
-
- Rogerson T. et al. Synaptic tagging during memory allocation. Nat Rev Neurosci 15, 157–169 (2014). https://doi.org:10.1038/nrn3667 - DOI - PMC - PubMed
-
- Josselyn S. A. & Frankland P. W. Memory Allocation: Mechanisms and Function. Annu Rev Neurosci 41, 389–413 (2018). https://doi.org:10.1146/annurev-neuro-080317-061956 - DOI - PMC - PubMed
Methods References
-
- Cai D. J. et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature 534, 115–118 (2016). https://doi.org:10.1038/nature17955 - DOI - PMC - PubMed
-
- Pennington Z. T. et al. ezTrack: An open-source video analysis pipeline for the investigation of animal behavior. Sci Rep 9, 19979 (2019). https://doi.org:10.1038/s41598-019-56408-9 - DOI - PMC - PubMed
-
- Dong Z. et al. Minian, an open-source miniscope analysis pipeline. Elife 11 (2022). https://doi.org:10.7554/eLife.70661 - DOI - PMC - PubMed
-
- Sheintuch L. et al. Tracking the Same Neurons across Multiple Days in Ca(2+) Imaging Data. Cell Rep 21, 1102–1115 (2017). https://doi.org:10.1016/j.celrep.2017.10.013 - DOI - PMC - PubMed
-
- Rubel O. et al. The Neurodata Without Borders ecosystem for neurophysiological data science. Elife 11 (2022). https://doi.org:10.7554/eLife.78362 - DOI - PMC - PubMed
Publication types
Grants and funding
- R01 NS116357/NS/NINDS NIH HHS/United States
- R01 AG013622/AG/NIA NIH HHS/United States
- R03 NS111493/NS/NINDS NIH HHS/United States
- R01 MH120162/MH/NIMH NIH HHS/United States
- R56 MH132959/MH/NIMH NIH HHS/United States
- RF1 AG072497/AG/NIA NIH HHS/United States
- R01 MH113071/MH/NIMH NIH HHS/United States
- F31 MH126543/MH/NIMH NIH HHS/United States
- DP2 MH122399/MH/NIMH NIH HHS/United States
- R21 DA049568/DA/NIDA NIH HHS/United States
- F32 NS116416/NS/NINDS NIH HHS/United States
- K99 MH131792/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
