This is a preprint.
Spinal sensorimotor circuits play a prominent role in hindlimb locomotor recovery after staggered thoracic lateral hemisections but cannot restore posture and interlimb coordination during quadrupedal locomotion in adult cats
- PMID: 36993268
- PMCID: PMC10055434
- DOI: 10.1101/2023.03.23.533936
Spinal sensorimotor circuits play a prominent role in hindlimb locomotor recovery after staggered thoracic lateral hemisections but cannot restore posture and interlimb coordination during quadrupedal locomotion in adult cats
Update in
-
Spinal Sensorimotor Circuits Play a Prominent Role in Hindlimb Locomotor Recovery after Staggered Thoracic Lateral Hemisections but Cannot Restore Posture and Interlimb Coordination during Quadrupedal Locomotion in Adult Cats.eNeuro. 2023 Jun 23;10(6):ENEURO.0191-23.2023. doi: 10.1523/ENEURO.0191-23.2023. Print 2023 Jun. eNeuro. 2023. PMID: 37328297 Free PMC article.
Abstract
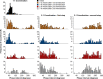
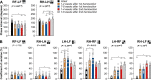
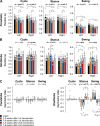
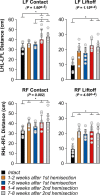
Spinal sensorimotor circuits interact with supraspinal and peripheral inputs to generate quadrupedal locomotion. Ascending and descending spinal pathways ensure coordination between the fore-and hindlimbs. Spinal cord injury disrupts these pathways. To investigate the control of interlimb coordination and hindlimb locomotor recovery, we performed two lateral thoracic hemisections placed on opposite sides of the cord (right T5-T6 and left T10-T11) at an interval of approximately two months in eight adult cats. In three cats, we then made a complete spinal transection caudal to the second hemisection at T12-T13. We collected electromyography and kinematic data during quadrupedal and hindlimb-only locomotion before and after spinal lesions. We show that 1) cats spontaneously recover quadrupedal locomotion following staggered hemisections but require balance assistance after the second one, 2) coordination between the fore-and hindlimbs displays 2:1 patterns and becomes weaker and more variable after both hemisections, 3) left-right asymmetries in hindlimb stance and swing durations appear after the first hemisection and reverse after the second, and 4) support periods reorganize after staggered hemisections to favor support involving both forelimbs and diagonal limbs. Cats expressed hindlimb locomotion the day following spinal transection, indicating that lumbar sensorimotor circuits play a prominent role in hindlimb locomotor recovery after staggered hemisections. These results reflect a series of changes in spinal sensorimotor circuits that allow cats to maintain and recover some level of quadrupedal locomotor functionality with diminished motor commands from the brain and cervical cord, although the control of posture and interlimb coordination remains impaired.
Significance statement: Coordinating the limbs during locomotion depends on pathways in the spinal cord. We used a spinal cord injury model that disrupts communication between the brain and spinal cord by sectioning half of the spinal cord on one side and then about two months later, half the spinal cord on the other side at different levels of the thoracic cord in cats. We show that despite a strong contribution from neural circuits located below the second spinal cord injury in the recovery of hindlimb locomotion, the coordination between the forelimbs and hindlimbs weakens and postural control is impaired. We can use our model to test approaches to restore the control of interlimb coordination and posture during locomotion after spinal cord injury.
Conflict of interest statement
Figures






References
-
- Alluin O, Karimi-Abdolrezaee S, Delivet-Mongrain H, Leblond H, Fehlings MG, Rossignol S (2011) Kinematic study of locomotor recovery after spinal cord clip compression injury in rats. J Neurotrauma 28:1963–1981. - PubMed
-
- Ballermann M, Fouad K (2006) Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur J Neurosci 23:1988–1996. - PubMed
-
- Ballion B, Morin D, Viala D (2001) Forelimb locomotor generators and quadrupedal locomotion in the neonatal rat. Eur J Neurosci 14:1727–1738. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
