This is a preprint.
Endocytic myosin-1 is a force-insensitive, power-generating motor
- PMID: 36993306
- PMCID: PMC10055380
- DOI: 10.1101/2023.03.21.533689
Endocytic myosin-1 is a force-insensitive, power-generating motor
Update in
-
Endocytic myosin-1 is a force-insensitive, power-generating motor.J Cell Biol. 2023 Oct 2;222(10):e202303095. doi: 10.1083/jcb.202303095. Epub 2023 Aug 7. J Cell Biol. 2023. PMID: 37549220 Free PMC article.
Abstract
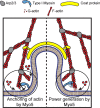
Myosins are required for clathrin-mediated endocytosis, but their precise molecular roles in this process are not known. This is, in part, because the biophysical properties of the relevant motors have not been investigated. Myosins have diverse mechanochemical activities, ranging from powerful contractility against mechanical loads to force-sensitive anchoring. To better understand the essential molecular contribution of myosin to endocytosis, we studied the in vitro force-dependent kinetics of the Saccharomyces cerevisiae endocytic type I myosin called Myo5, a motor whose role in clathrin-mediated endocytosis has been meticulously studied in vivo. We report that Myo5 is a low-duty-ratio motor that is activated ∼10-fold by phosphorylation, and that its working stroke and actin-detachment kinetics are relatively force-insensitive. Strikingly, the in vitro mechanochemistry of Myo5 is more like that of cardiac myosin than like that of slow anchoring myosin-1s found on endosomal membranes. We therefore propose that Myo5 generates power to augment actin assembly-based forces during endocytosis in cells.
Summary: Pedersen, Snoberger et al. measure the force-sensitivity of the yeast endocytic the myosin-1 called Myo5 and find that it is more likely to generate power than to serve as a force-sensitive anchor in cells. Implications for Myo5's role in clathrin-mediated endocytosis are discussed.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
