This is a preprint.
Enveloped viruses pseudotyped with mammalian myogenic cell fusogens target skeletal muscle for gene delivery
- PMID: 36993357
- PMCID: PMC10055243
- DOI: 10.1101/2023.03.17.533157
Enveloped viruses pseudotyped with mammalian myogenic cell fusogens target skeletal muscle for gene delivery
Update in
-
Enveloped viruses pseudotyped with mammalian myogenic cell fusogens target skeletal muscle for gene delivery.Cell. 2023 May 11;186(10):2062-2077.e17. doi: 10.1016/j.cell.2023.03.033. Epub 2023 Apr 18. Cell. 2023. PMID: 37075755 Free PMC article.
Abstract
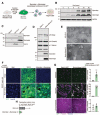
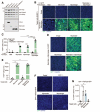
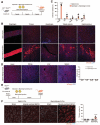
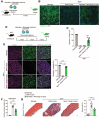
Entry of enveloped viruses into cells is mediated by fusogenic proteins that form a complex between membranes to drive rearrangements needed for fusion. Skeletal muscle development also requires membrane fusion events between progenitor cells to form multinucleated myofibers. Myomaker and Myomerger are muscle-specific cell fusogens, but do not structurally or functionally resemble classical viral fusogens. We asked if the muscle fusogens could functionally substitute for viral fusogens, despite their structural distinctiveness, and fuse viruses to cells. We report that engineering of Myomaker and Myomerger on the membrane of enveloped viruses leads to specific transduction of skeletal muscle. We also demonstrate that locally and systemically injected virions pseudotyped with the muscle fusogens can deliver micro-Dystrophin (μDys) to skeletal muscle of a mouse model of Duchenne muscular dystrophy. Through harnessing the intrinsic properties of myogenic membranes, we establish a platform for delivery of therapeutic material to skeletal muscle.
Conflict of interest statement
Declaration of interests
The authors declare competing financial interests: S.M.H. and D.P.M. have filed patent applications on this work through Cincinnati Children’s Hospital Medical Center.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
