This is a preprint.
Stability and heterogeneity in the anti-microbiota reactivity of human milk-derived Immunoglobulin A
- PMID: 36993366
- PMCID: PMC10055037
- DOI: 10.1101/2023.03.16.532940
Stability and heterogeneity in the anti-microbiota reactivity of human milk-derived Immunoglobulin A
Update in
-
Stability and heterogeneity in the antimicrobiota reactivity of human milk-derived immunoglobulin A.J Exp Med. 2023 Aug 7;220(8):e20220839. doi: 10.1084/jem.20220839. Epub 2023 Jul 18. J Exp Med. 2023. PMID: 37462916 Free PMC article.
Abstract
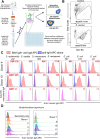
Immunoglobulin A (IgA) is secreted into breast milk and is critical to both protecting against enteric pathogens and shaping the infant intestinal microbiota. The efficacy of breast milk-derived maternal IgA (BrmIgA) is dependent upon its specificity, however heterogeneity in BrmIgA binding ability to the infant microbiota is not known. Using a flow cytometric array, we analyzed the reactivity of BrmIgA against bacteria common to the infant microbiota and discovered substantial heterogeneity between all donors, independent of preterm or term delivery. We also observed intra-donor variability in the BrmIgA response to closely related bacterial isolates. Conversely, longitudinal analysis showed that the anti-bacterial BrmIgA reactivity was relatively stable through time, even between sequential infants, indicating that mammary gland IgA responses are durable. Together, our study demonstrates that the anti-bacterial BrmIgA reactivity displays inter-individual heterogeneity but intra-individual stability. These findings have important implications for how breast milk shapes the development of the infant microbiota and protects against Necrotizing Enterocolitis.
Summary: We analyze the ability of breast milk-derived Immunoglobulin A (IgA) antibodies to bind the infant intestinal microbiota. We discover that each mother secretes into their breast milk a distinct set of IgA antibodies that are stably maintained over time.
Figures





References
-
- Ackerman M.E., Das J., Pittala S., Broge T., Linde C., Suscovich T.J., Brown E.P., Bradley T., Natarajan H., Lin S., Sassic J.K., O’Keefe S., Mehta N., Goodman D., Sips M., Weiner J.A., Tomaras G.D., Haynes B.F., Lauffenburger D.A., Bailey-Kellogg C., Roederer M., and Alter G.. 2018. Route of immunization defines multiple mechanisms of vaccine-mediated protection against SIV. Nat Med 24:1590–1598. - PMC - PubMed
-
- Adhisivam B., Vishnu Bhat B., Rao K., Kingsley S.M., Plakkal N., and Palanivel C.. 2018. Effect of Holder pasteurization on macronutrients and immunoglobulin profile of pooled donor human milk. J Matern Fetal Neonatal Med 1–4. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
