This is a preprint.
Statistical Challenges when Analyzing SARS-CoV-2 RNA Measurements Below the Assay Limit of Quantification in COVID-19 Clinical Trials
- PMID: 36993419
- PMCID: PMC10055451
- DOI: 10.1101/2023.03.13.23287208
Statistical Challenges when Analyzing SARS-CoV-2 RNA Measurements Below the Assay Limit of Quantification in COVID-19 Clinical Trials
Update in
-
Statistical Challenges When Analyzing SARS-CoV-2 RNA Measurements Below the Assay Limit of Quantification in COVID-19 Clinical Trials.J Infect Dis. 2023 Aug 31;228(Suppl 2):S101-S110. doi: 10.1093/infdis/jiad285. J Infect Dis. 2023. PMID: 37650235 Free PMC article. Clinical Trial.
Abstract
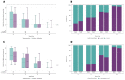
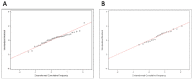
Most clinical trials evaluating COVID-19 therapeutics include assessments of antiviral activity. In recently completed outpatient trials, changes in nasal SARS-CoV-2 RNA levels from baseline were commonly assessed using analysis of covariance (ANCOVA) or mixed models for repeated measures (MMRM) with single-imputation for results below assay lower limits of quantification (LLoQ). Analyzing changes in viral RNA levels with singly-imputed values can lead to biased estimates of treatment effects. In this paper, using an illustrative example from the ACTIV-2 trial, we highlight potential pitfalls of imputation when using ANCOVA or MMRM methods, and illustrate how these methods can be used when considering values <LLoQ as censored measurements. Best practices when analyzing quantitative viral RNA data should include details about the assay and its LLoQ, completeness summaries of viral RNA data, and outcomes among participants with baseline viral RNA ≥LLoQ, as well as those with viral RNA <LLoQ.
Figures
References
-
- COVID-19: Developing Drugs and Biological Products for Treatment or Prevention. 2021; .
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
