This is a preprint.
Tumour mitochondrial DNA mutations drive aerobic glycolysis to enhance checkpoint blockade
- PMID: 36993533
- PMCID: PMC10055208
- DOI: 10.1101/2023.03.21.533091
Tumour mitochondrial DNA mutations drive aerobic glycolysis to enhance checkpoint blockade
Update in
-
Mitochondrial DNA mutations drive aerobic glycolysis to enhance checkpoint blockade response in melanoma.Nat Cancer. 2024 Apr;5(4):659-672. doi: 10.1038/s43018-023-00721-w. Epub 2024 Jan 29. Nat Cancer. 2024. PMID: 38286828 Free PMC article.
Abstract
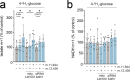
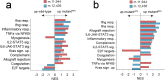
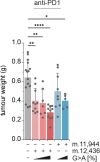
The mitochondrial genome encodes essential machinery for respiration and metabolic homeostasis but is paradoxically among the most common targets of somatic mutation in the cancer genome, with truncating mutations in respiratory complex I genes being most over-represented1. While mitochondrial DNA (mtDNA) mutations have been associated with both improved and worsened prognoses in several tumour lineages1-3, whether these mutations are drivers or exert any functional effect on tumour biology remains controversial. Here we discovered that complex I-encoding mtDNA mutations are sufficient to remodel the tumour immune landscape and therapeutic resistance to immune checkpoint blockade. Using mtDNA base editing technology4 we engineered recurrent truncating mutations in the mtDNA-encoded complex I gene, Mt-Nd5, into murine models of melanoma. Mechanistically, these mutations promoted utilisation of pyruvate as a terminal electron acceptor and increased glycolytic flux without major effects on oxygen consumption, driven by an over-reduced NAD pool and NADH shuttling between GAPDH and MDH1, mediating a Warburg-like metabolic shift. In turn, without modifying tumour growth, this altered cancer cell-intrinsic metabolism reshaped the tumour microenvironment in both mice and humans, promoting an anti-tumour immune response characterised by loss of resident neutrophils. This subsequently sensitised tumours bearing high mtDNA mutant heteroplasmy to immune checkpoint blockade, with phenocopy of key metabolic changes being sufficient to mediate this effect. Strikingly, patient lesions bearing >50% mtDNA mutation heteroplasmy also demonstrated a >2.5-fold improved response rate to checkpoint inhibitor blockade. Taken together these data nominate mtDNA mutations as functional regulators of cancer metabolism and tumour biology, with potential for therapeutic exploitation and treatment stratification.
Conflict of interest statement
Competing interests M.M., E.R. and P.A.G. are named inventors on patent applications resulting from this work filed by Cancer Research Horizons. P.A.G is a shareholder, and has been a consultant and Scientific Advisory Board member to Pretzel Therapeutics Inc.
Figures
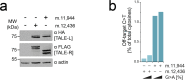

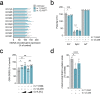
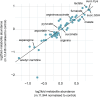
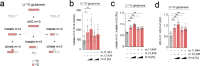












References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
