This is a preprint.
Structural basis for functional properties of cytochrome c oxidase
- PMID: 36993562
- PMCID: PMC10055264
- DOI: 10.1101/2023.03.20.530986
Structural basis for functional properties of cytochrome c oxidase
Abstract
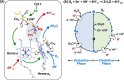
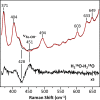
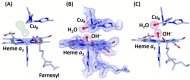
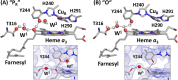
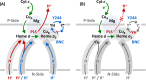
Cytochrome c oxidase (CcO) is an essential enzyme in mitochondrial and bacterial respiration. It catalyzes the four-electron reduction of molecular oxygen to water and harnesses the chemical energy to translocate four protons across biological membranes, thereby establishing the proton gradient required for ATP synthesis1. The full turnover of the CcO reaction involves an oxidative phase, in which the reduced enzyme (R) is oxidized by molecular oxygen to the metastable oxidized OH state, and a reductive phase, in which OH is reduced back to the R state. During each of the two phases, two protons are translocated across the membranes2. However, if OH is allowed to relax to the resting oxidized state (O), a redox equivalent to OH, its subsequent reduction to R is incapable of driving proton translocation2,3. How the O state structurally differs from OH remains an enigma in modern bioenergetics. Here, with resonance Raman spectroscopy and serial femtosecond X-ray crystallography (SFX)4, we show that the heme a3 iron and CuB in the active site of the O state, like those in the OH state5,6, are coordinated by a hydroxide ion and a water molecule, respectively. However, Y244, a residue covalently linked to one of the three CuB ligands and critical for the oxygen reduction chemistry, is in the neutral protonated form, which distinguishes O from OH, where Y244 is in the deprotonated tyrosinate form. These structural characteristics of O provide new insights into the proton translocation mechanism of CcO.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
