This is a preprint.
Atlas of Plasmodium falciparum intraerythrocytic development using expansion microscopy
- PMID: 36993606
- PMCID: PMC10055389
- DOI: 10.1101/2023.03.22.533773
Atlas of Plasmodium falciparum intraerythrocytic development using expansion microscopy
Update in
-
Atlas of Plasmodium falciparum intraerythrocytic development using expansion microscopy.Elife. 2023 Dec 18;12:RP88088. doi: 10.7554/eLife.88088. Elife. 2023. PMID: 38108809 Free PMC article.
Abstract
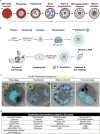
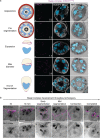
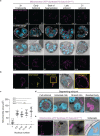
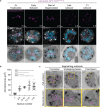
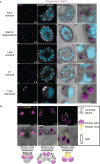
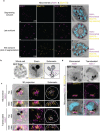
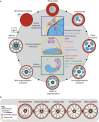
Apicomplexan parasites exhibit tremendous diversity in much of their fundamental cell biology, but study of these organisms using light microscopy is often hindered by their small size. Ultrastructural expansion microscopy (U-ExM) is a microscopy preparation method that physically expands the sample ~4.5x. Here, we apply U-ExM to the human malaria parasite Plasmodium falciparum during the asexual blood stage of its lifecycle to understand how this parasite is organized in three-dimensions. Using a combination of dye-conjugated reagents and immunostaining, we have catalogued 13 different P. falciparum structures or organelles across the intraerythrocytic development of this parasite and made multiple observations about fundamental parasite cell biology. We describe that the outer centriolar plaque and its associated proteins anchor the nucleus to the parasite plasma membrane during mitosis. Furthermore, the rhoptries, Golgi, basal complex, and inner membrane complex, which form around this anchoring site while nuclei are still dividing, are concurrently segregated and maintain an association to the outer centriolar plaque until the start of segmentation. We also show that the mitochondrion and apicoplast undergo sequential fission events while maintaining an association with the outer centriolar plaque during cytokinesis. Collectively, this study represents the most detailed ultrastructural analysis of P. falciparum during its intraerythrocytic development to date, and sheds light on multiple poorly understood aspects of its organelle biogenesis and fundamental cell biology.
Conflict of interest statement
COMPETING INTERESTS The authors declare no competing interests.
Figures



References
Publication types
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
