This is a preprint.
Central circuit controlling thermoregulatory inversion and torpor-like state
- PMID: 36993654
- PMCID: PMC10055657
- DOI: 10.21203/rs.3.rs-2698203/v1
Central circuit controlling thermoregulatory inversion and torpor-like state
Abstract
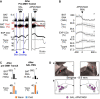
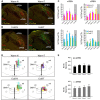
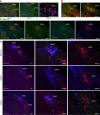
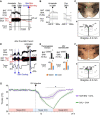
To maintain core body temperature in mammals, the CNS thermoregulatory networks respond to cold exposure by increasing brown adipose tissue and shivering thermogenesis. However, in hibernation or torpor, this normal thermoregulatory response is supplanted by "thermoregulatory inversion", an altered homeostatic state in which cold exposure causes inhibition of thermogenesis and warm exposure stimulates thermogenesis. Here we demonstrate the existence of a novel, dynorphinergic thermoregulatory reflex pathway between the dorsolateral parabrachial nucleus and the dorsomedial hypothalamus that bypasses the normal thermoregulatory integrator in the hypothalamic preoptic area to play a critical role in mediating the inhibition of thermogenesis during thermoregulatory inversion. Our results indicate the existence of a neural circuit mechanism for thermoregulatory inversion within the CNS thermoregulatory pathways and support the potential for inducing a homeostatically-regulated, therapeutic hypothermia in non-hibernating species, including humans.
Conflict of interest statement
Competing interests No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

