This is a preprint.
Two-fluid dynamics and micron-thin boundary layers shape cytoplasmic flows in early Drosophila embryos
- PMID: 36993669
- PMCID: PMC10055070
- DOI: 10.1101/2023.03.16.532979
Two-fluid dynamics and micron-thin boundary layers shape cytoplasmic flows in early Drosophila embryos
Update in
-
Two-fluid dynamics and micron-thin boundary layers shape cytoplasmic flows in early Drosophila embryos.Proc Natl Acad Sci U S A. 2023 Oct 31;120(44):e2302879120. doi: 10.1073/pnas.2302879120. Epub 2023 Oct 25. Proc Natl Acad Sci U S A. 2023. PMID: 37878715 Free PMC article.
Abstract
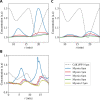
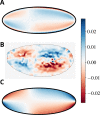
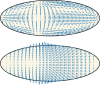
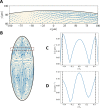
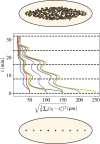
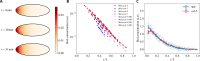
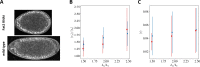
Cytoplasmic flows are widely emerging as key functional players in development. In early Drosophila embryos, flows drive the spreading of nuclei across the embryo. Here, we combine hydrodynamic modeling with quantitative imaging to develop a two-fluid model that features an active actomyosin gel and a passive viscous cytosol. Gel contractility is controlled by the cell cycle oscillator, the two fluids being coupled by friction. In addition to recapitulating experimental flow patterns, our model explains observations that remained elusive, and makes a series of new predictions. First, the model captures the vorticity of cytosolic flows, which highlights deviations from Stokes' flow that were observed experimentally but remained unexplained. Second, the model reveals strong differences in the gel and cytosol motion. In particular, a micron-sized boundary layer is predicted close to the cortex, where the gel slides tangentially whilst the cytosolic flow cannot slip. Third, the model unveils a mechanism that stabilizes the spreading of nuclei with respect to perturbations of their initial positions. This self-correcting mechanism is argued to be functionally important for proper nuclear spreading. Fourth, we use our model to analyze the effects of flows on the transport of the morphogen Bicoid, and the establishment of its gradients. Finally, the model predicts that the flow strength should be reduced if the shape of the domain is more round, which is experimentally confirmed in Drosophila mutants. Thus, our two-fluid model explains flows and nuclear positioning in early Drosophila, while making predictions that suggest novel future experiments.
Figures

Similar articles
-
Two-fluid dynamics and micron-thin boundary layers shape cytoplasmic flows in early Drosophila embryos.Proc Natl Acad Sci U S A. 2023 Oct 31;120(44):e2302879120. doi: 10.1073/pnas.2302879120. Epub 2023 Oct 25. Proc Natl Acad Sci U S A. 2023. PMID: 37878715 Free PMC article.
-
Cortex-driven cytoplasmic flows in elongated cells: fluid mechanics and application to nuclear transport in Drosophila embryos.J R Soc Interface. 2023 Nov;20(208):20230428. doi: 10.1098/rsif.2023.0428. Epub 2023 Nov 15. J R Soc Interface. 2023. PMID: 37963561 Free PMC article.
-
Self-Organized Nuclear Positioning Synchronizes the Cell Cycle in Drosophila Embryos.Cell. 2019 May 2;177(4):925-941.e17. doi: 10.1016/j.cell.2019.03.007. Epub 2019 Apr 11. Cell. 2019. PMID: 30982601 Free PMC article.
-
Aspects of fluid dynamics applied to the larger arteries.J Theor Biol. 1999 Jul 21;199(2):137-61. doi: 10.1006/jtbi.1999.0953. J Theor Biol. 1999. PMID: 10395811 Review.
-
Reshaping the Syncytial Drosophila Embryo with Cortical Actin Networks: Four Main Steps of Early Development.Results Probl Cell Differ. 2024;71:67-90. doi: 10.1007/978-3-031-37936-9_4. Results Probl Cell Differ. 2024. PMID: 37996673 Review.
References
-
- Seifriz W., Mechanism of Protoplasmic Movement, Nature 171, 1136 (1953). - PubMed
-
- Stewart P. A., The Organization of Movement in Slime Mold Plasmodia, in Primitive Motile Systems in Cell Biology (Second Edition), edited by Allen R. D. and Kamiya N. (Academic Press, 1964) pp. 69–78.
-
- Nonaka S., Tanaka Y., Okada Y., Takeda S., Harada A., Kanai Y., Kido M., and Hirokawa N., Randomization of Left–Right Asymmetry due to Loss of Nodal Cilia Generating Leftward Flow of Extraembryonic Fluid in Mice Lacking KIF3B Motor Protein, Cell 95, 829 (1998). - PubMed
-
- Hirokawa N., Tanaka Y., Okada Y., and Takeda S., Nodal Flow and the Generation of Left-Right Asymmetry, Cell 125, 33 (2006). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
