This is a preprint.
Ganglioside GD3 regulates neural stem cell quiescence and controls postnatal neurogenesis
- PMID: 36993675
- PMCID: PMC10055067
- DOI: 10.1101/2023.03.14.532547
Ganglioside GD3 regulates neural stem cell quiescence and controls postnatal neurogenesis
Update in
-
Ganglioside GD3 regulates neural stem cell quiescence and controls postnatal neurogenesis.Glia. 2024 Jan;72(1):167-183. doi: 10.1002/glia.24468. Epub 2023 Sep 5. Glia. 2024. PMID: 37667994 Free PMC article.
Abstract
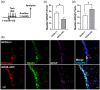
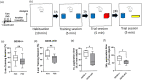
The postnatal neural stem cell (NSC) pool hosts quiescent and activated radial glia-like NSCs contributing to neurogenesis throughout adulthood. However, the underlying regulatory mechanism during the transition from quiescent NSCs to activated NSCs in the postnatal NSC niche is not fully understood. Lipid metabolism and lipid composition play important roles in regulating NSC fate determination. Biological lipid membranes define the individual cellular shape and help maintain cellular organization and are highly heterogenous in structure and there exist diverse microdomains (also known as lipid rafts), which are enriched with sugar molecules, such as glycosphingolipids. An often overlooked but key aspect is that the functional activities of proteins and genes are highly dependent upon their molecular environments. We previously reported that ganglioside GD3 is the predominant species in NSCs and that the reduced postnatal NSC pools are observed in global GD3-synthase knockout (GD3S-KO) mouse brains. The specific roles of GD3 in determining the stage and cell-lineage determination of NSCs remain unclear, since global GD3S-KO mice cannot distinguish if GD3 regulates postnatal neurogenesis or developmental impacts. Here we show that inducible GD3 deletion in postnatal radial glia-like NSCs promotes the NSC activation, resulting in the loss of the long-term maintenance of the adult NSC pools. The reduced neurogenesis in the subventricular zone (SVZ) and the dentate gyrus (DG) of GD3S-conditional-knockout mice led to impaired olfactory and memory functions. Thus, our results provide convincing evidence that postnatal GD3 maintains the quiescent state of radial glia-like NSCs in the adult NSC niche.
Conflict of interest statement
CONFLICT OF INTEREST
The authors have no relevant financial or non-financial interests to disclose.
Figures






References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
