The safety and immunogenicity of a two-dose schedule of CoronaVac, and the immune persistence of vaccination for six months, in people living with HIV: A multicenter prospective cohort study
- PMID: 36993947
- PMCID: PMC10040764
- DOI: 10.3389/fimmu.2023.1129651
The safety and immunogenicity of a two-dose schedule of CoronaVac, and the immune persistence of vaccination for six months, in people living with HIV: A multicenter prospective cohort study
Abstract
Background: People living with HIV (PLWH) are more vulnerable to SARS-CoV-2. However, evidence on the immunogenicity of coronavirus disease 2019 (COVID-19) vaccines in this population is insufficient. The objective of this study is to assess the immunogenicity and safety of the two-dose schedule of Sinovac CoronaVac for 6 months postvaccination in PLWH.
Methods: We conducted a multicenter prospective cohort study among PLWH and HIV-negative adults in China. Participants who received two doses of CoronaVac prior to the recruitment were allocated into two groups and followed up for 6 months. The neutralizing antibodies (nAbs), immunoglobulin G against the receptor-binding domain of the spike protein (S-IgG), and gamma-interferon (IFN-γ) were measured to assess the associations among CoronaVac immunogenicity and related factors. Adverse reactions were collected to evaluate the safety profile of vaccination.
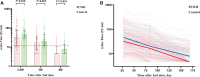
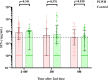
Results: A total of 203 PLWH and 100 HIV-negative individuals were enrolled. A small portion of participants reported mild or moderate adverse reactions without serious adverse events. Median nAbs level in PLWH (31.96 IU/mL, IQR: 12.34-76.40) was lower than that in the control group (46.52 IU/mL, IQR: 29.08-77.30) at the 2-4 weeks postvaccination (P=0.002), and the same trend was presented for median S-IgG titer (37.09 vs. 60.02 IU/ml) (both P <0.05). The nAbs seroconversion rate in the PLWH group was also lower than in the control group (75.86% vs. 89.00%). After then, the immune responses reduced over time in term of only 23.04% of PLWH and 36.00% of HIV-negative individuals had a positive seroconversion for nAbs at 6-month. The multivariable generalized estimating equation analysis showed that PLWH with CD4+T count≥350 cells/µL presented higher immune response than PLWH with CD4+T count <350 cells/µL in terms of antibody seroconversion and titers. The immunogenicity did not differ in participants with low or high HIV viral load. The S-antigen specific IFN-γ immunity was generally stable and had a slow attenuation in both two groups for 6 months postvaccination.
Conclusion: The Sinovac CoronaVac was generally safe and immunogenic in PLWH, but the immunity response was inferior and the antibodies vanished faster compared to HIV-negative individuals. This study suggested a shorter than 6-month interval of prime-boost vaccination for PLWH to ensure a better protection.
Keywords: PLWH; S-IgG antibody; Sinovac CoronaVac; adverse events; neutralizing antibody.
Copyright © 2023 Wang, Qiao, Huo, Wang, Liang, Yu, Lan, Song, Zhang, Yan and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Johns Hopkins University . COVID-19 dashboard by the center for systems science and engineering (CSSE) at johns Hopkins university (JHU) (2022). Available at: https://coronavirus.jhu.edu/map.html.
-
- World Health Organization . HIV Data and statistics (2022). Available at: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/s....
-
- Grenfell RFQ, Almeida NBF, Filgueiras PS, Corsini CA, Gomes SVC, de Miranda DAP, et al. Immunogenicity, effectiveness, and safety of inactivated virus (CoronaVac) vaccine in a two-dose primary protocol and BNT162b2 heterologous booster in Brazil (Immunita-001): A one year period follow up phase 4 study. Front Immunol (2022) 13:918896. doi: 10.3389/fimmu.2022.918896 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

