Neutrophil extracellular traps in tumor progression and immunotherapy
- PMID: 36993957
- PMCID: PMC10040667
- DOI: 10.3389/fimmu.2023.1135086
Neutrophil extracellular traps in tumor progression and immunotherapy
Abstract
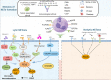
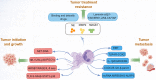
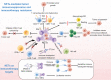
Tumor immunity is a growing field of research that involves immune cells within the tumor microenvironment. Neutrophil extracellular traps (NETs) are neutrophil-derived extracellular web-like chromatin structures that are composed of histones and granule proteins. Initially discovered as the predominant host defense against pathogens, NETs have attracted increasing attention due to they have also been tightly associated with tumor. Excessive NET formation has been linked to increased tumor growth, metastasis, and drug resistance. Moreover, through direct and/or indirect effects on immune cells, an abnormal increase in NETs benefits immune exclusion and inhibits T-cell mediated antitumor immune responses. In this review, we summarize the recent but rapid progress in understanding the pivotal roles of NETs in tumor and anti-tumor immunity, highlighting the most relevant challenges in the field. We believe that NETs may be a promising therapeutic target for tumor immunotherapy.
Keywords: anti-tumor immunity; immunotherapy; neutrophil extracellular traps; tumor microenvironment; tumor progression.
Copyright © 2023 Yan, Gu, Sun and Ge.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
No NETs no TIME: Crosstalk between neutrophil extracellular traps and the tumor immune microenvironment.Front Immunol. 2022 Dec 23;13:1075260. doi: 10.3389/fimmu.2022.1075260. eCollection 2022. Front Immunol. 2022. PMID: 36618417 Free PMC article. Review.
-
NETworking with cancer: The bidirectional interplay between cancer and neutrophil extracellular traps.Cancer Cell. 2023 Mar 13;41(3):505-526. doi: 10.1016/j.ccell.2023.02.001. Epub 2023 Feb 23. Cancer Cell. 2023. PMID: 36827980 Free PMC article. Review.
-
The Emerging Role of Neutrophil Extracellular Traps (NETs) in Tumor Progression and Metastasis.Front Immunol. 2020 Sep 16;11:1749. doi: 10.3389/fimmu.2020.01749. eCollection 2020. Front Immunol. 2020. PMID: 33042107 Free PMC article. Review.
-
Neutrophil Extracellular Traps: A New Player in Cancer Metastasis and Therapeutic Target.J Exp Clin Cancer Res. 2021 Jul 16;40(1):233. doi: 10.1186/s13046-021-02013-6. J Exp Clin Cancer Res. 2021. PMID: 34271947 Free PMC article. Review.
-
Neutrophil Extracellular Traps and Cancer: Trapping Our Attention with Their Involvement in Ovarian Cancer.Int J Mol Sci. 2023 Mar 22;24(6):5995. doi: 10.3390/ijms24065995. Int J Mol Sci. 2023. PMID: 36983067 Free PMC article. Review.
Cited by
-
A Web of Challenges: The Therapeutic Struggle to Target NETs in Disease.Int J Mol Sci. 2025 May 16;26(10):4773. doi: 10.3390/ijms26104773. Int J Mol Sci. 2025. PMID: 40429915 Free PMC article. Review.
-
The crucial regulatory role of type I interferon in inflammatory diseases.Cell Biosci. 2023 Dec 20;13(1):230. doi: 10.1186/s13578-023-01188-z. Cell Biosci. 2023. PMID: 38124132 Free PMC article. Review.
-
TRIM8 as a predictor for prognosis in childhood acute lymphoblastic leukemia based on a signature of neutrophil extracellular traps.Front Oncol. 2024 Aug 19;14:1427776. doi: 10.3389/fonc.2024.1427776. eCollection 2024. Front Oncol. 2024. PMID: 39224802 Free PMC article.
-
Neutrophils and Neutrophil-Based Drug Delivery Systems in Anti-Cancer Therapy.Cancers (Basel). 2025 Apr 5;17(7):1232. doi: 10.3390/cancers17071232. Cancers (Basel). 2025. PMID: 40227814 Free PMC article. Review.
-
Identification of neutrophil extracellular traps genes as potential biomarkers in psoriasis based on bioinformatics analysis.Sci Rep. 2024 Oct 11;14(1):23848. doi: 10.1038/s41598-024-75069-x. Sci Rep. 2024. PMID: 39394253 Free PMC article.
References
-
- Banta KL, Xu X, Chitre AS, Au-Yeung A, Takahashi C, O'Gorman WE, et al. . Mechanistic convergence of the TIGIT and PD-1 inhibitory pathways necessitates co-blockade to optimize anti-tumor CD8(+) T cell responses. Immunity (2022) 55(3):512–26.e9. doi: 10.1016/j.immuni.2022.02.005 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

