Sirtuin-dependent metabolic and epigenetic regulation of macrophages during tuberculosis
- PMID: 36993975
- PMCID: PMC10040548
- DOI: 10.3389/fimmu.2023.1121495
Sirtuin-dependent metabolic and epigenetic regulation of macrophages during tuberculosis
Abstract
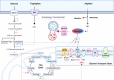
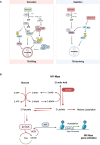
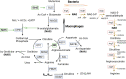
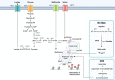
Macrophages are the preeminent phagocytic cells which control multiple infections. Tuberculosis a leading cause of death in mankind and the causative organism Mycobacterium tuberculosis (MTB) infects and persists in macrophages. Macrophages use reactive oxygen and nitrogen species (ROS/RNS) and autophagy to kill and degrade microbes including MTB. Glucose metabolism regulates the macrophage-mediated antimicrobial mechanisms. Whereas glucose is essential for the growth of cells in immune cells, glucose metabolism and its downsteam metabolic pathways generate key mediators which are essential co-substrates for post-translational modifications of histone proteins, which in turn, epigenetically regulate gene expression. Herein, we describe the role of sirtuins which are NAD+-dependent histone histone/protein deacetylases during the epigenetic regulation of autophagy, the production of ROS/RNS, acetyl-CoA, NAD+, and S-adenosine methionine (SAM), and illustrate the cross-talk between immunometabolism and epigenetics on macrophage activation. We highlight sirtuins as emerging therapeutic targets for modifying immunometabolism to alter macrophage phenotype and antimicrobial function.
Keywords: SIRTUIN; autophagy; glycolysis; histone modifications; human macrophages; metabolism.
Copyright © 2023 Zhang, Sowers, Cherryhomes, Singh, Mishra, Restrepo, Khan and Jagannath.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

