A coumarin-based fluorescent chemosensor as a Sn indicator and a fluorescent cellular imaging agent
- PMID: 36994144
- PMCID: PMC10041825
- DOI: 10.1039/d2ra07884h
A coumarin-based fluorescent chemosensor as a Sn indicator and a fluorescent cellular imaging agent
Abstract
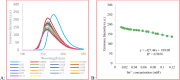
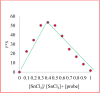
In the present study, fluorogenic coumarin-based probes (1-3) through condensation of 4-hydroxy coumarin with malondialdehyde bis(diethyl acetal)/triethyl orthoformate were prepared. The absorption and fluorescence emission properties of 2b and 3 in different solvents were studied, and a considerable solvatochromic effect was observed. The sensitivity of chemosensors 2b and 3 toward various cations and anions was investigated. It was revealed that compound 3 had a distinct selectivity toward Sn2+, possibly via a chelation enhanced quenching mechanism. The fluorescence signal was quenched over the concentration range of 6.6-120 μM, with an LOD value of 3.89 μM. The cytotoxicity evaluation of 3 against breast cancer cell lines demonstrated that the chemosensor was nontoxic and could be used successfully in cellular imaging. The probe responded to tin ions not only via fluorescence quenching, but also through colorimetric signal change. The change in optical properties was observed in ambient conditions and inside living cells.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
All authors declare no conflict of interest.
Figures









References
-
- Conti M. E., in Mineral Components in Foods, CRC press, 2006, pp. 339–362
-
- Hoch M. Appl. Geochem. 2001;16:719–743.
-
- Rajan I. Narayanan N. Rabindran R. Jayasree P. R. Manish Kumar P. R. Biol. Trace Elem. Res. 2013;155:455–459. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

