The skeletal muscle fiber periphery: A nexus of mTOR-related anabolism
- PMID: 36994172
- PMCID: PMC10040390
- DOI: 10.1016/j.smhs.2022.11.004
The skeletal muscle fiber periphery: A nexus of mTOR-related anabolism
Abstract
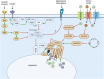
Skeletal muscle anabolism is driven by numerous stimuli such as growth factors, nutrients (i.e., amino acids, glucose), and mechanical stress. These stimuli are integrated by the mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) signal transduction cascade. In recent years, work from our laboratory and elsewhere has sought to unravel the molecular mechanisms underpinning the mTOR-related activation of muscle protein synthesis (MPS), as well as the spatial regulation of these mechanisms within the skeletal muscle cell. These studies have suggested that the skeletal muscle fiber periphery is a region of central importance in anabolism (i.e., growth/MPS). Indeed, the fiber periphery is replete with the substrates, molecular machinery, and translational apparatus necessary to facilitate MPS. This review provides a summary of the mechanisms underpinning the mTOR-associated activation of MPS from cell, rodent, and human studies. It also presents an overview of the spatial regulation of mTORC1 in response to anabolic stimuli and outlines the factors that distinguish the periphery of the cell as a highly notable region of skeletal muscle for the induction of MPS. Future research should seek to further explore the nutrient-induced activation of mTORC1 at the periphery of skeletal muscle fibers.
Keywords: Hypertrophy; Muscle protein synthesis; Periphery; Skeletal muscle; Translation; mTOR.
© 2022 Chengdu Sport University. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co. Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
