Advances in Unhealthy Nutrition and Circadian Dysregulation in Pathophysiology of NAFLD
- PMID: 36994336
- PMCID: PMC10012147
- DOI: 10.3389/fcdhc.2021.691828
Advances in Unhealthy Nutrition and Circadian Dysregulation in Pathophysiology of NAFLD
Abstract
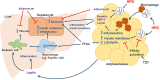
Unhealthy diets and lifestyle result in various metabolic conditions including metabolic syndrome and non-alcoholic fatty liver disease (NAFLD). Much evidence indicates that disruption of circadian rhythms contributes to the development and progression of excessive hepatic fat deposition and inflammation, as well as liver fibrosis, a key characteristic of non-steatohepatitis (NASH) or the advanced form of NAFLD. In this review, we emphasize the importance of nutrition as a critical factor in the regulation of circadian clock in the liver. We also focus on the roles of the rhythms of nutrient intake and the composition of diets in the regulation of circadian clocks in the context of controlling hepatic glucose and fat metabolism. We then summarize the effects of unhealthy nutrition and circadian dysregulation on the development of hepatic steatosis and inflammation. A better understanding of how the interplay among nutrition, circadian rhythms, and dysregulated metabolism result in hepatic steatosis and inflammation can help develop improved preventive and/or therapeutic strategies for managing NAFLD.
Keywords: NAFLD; circadian; hepatic steatosis; inflammation; metabolic diseases; nutrition.
Copyright © 2021 Guo, Zheng, Zhang, Jiang, Chen, Yu, Wang, Ma and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

