Osteoconductivity and neurotoxicity of silver-containing hydroxyapatite coating cage for spinal interbody fusion in rats
- PMID: 36994462
- PMCID: PMC10041372
- DOI: 10.1002/jsp2.1236
Osteoconductivity and neurotoxicity of silver-containing hydroxyapatite coating cage for spinal interbody fusion in rats
Abstract
Background: The use of spinal instrumentation is an established risk factor for postoperative infection. To address this problem, we prepared silver-containing hydroxyapatite coating, consisting of highly osteoconductive hydroxyapatite interfused with silver. The technology has been adopted for total hip arthroplasty. Silver-containing hydroxyapatite coating has been reported to have good biocompatibility and low toxicity. However, no studies about applying this coating in spinal surgery have addressed the osteoconductivity and direct neurotoxicity to the spinal cord of silver-containing hydroxyapatite cages in spinal interbody fusion.
Aim: In this study, we evaluated the osteoconductivity and neurotoxicity of silver-containing hydroxyapatite-coated implants in rats.
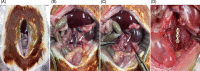
Materials & methods: Titanium (non-coated, hydroxyapatite-coated, and silver-containing hydroxyapatite-coated) interbody cages were inserted into the spine for anterior lumbar fusion. At 8 weeks postoperatively, micro-computed tomography and histology were performed to evaluate the osteoconductivity of the cage. Inclined plane test and toe pinch test were performed postoperatively to assess neurotoxicity.
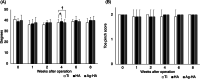
Results: Micro-computed tomography data indicated no significant difference in bone volume/total volume among the three groups. Histologically, the hydroxyapatite-coated and silver-containing hydroxyapatite-coated groups showed significantly higher bone contact rate than that of the titanium group. In contrast, there was no significant difference in bone formation rate among the three groups. Data of inclined plane and toe pinch test showed no significant loss of motor and sensory function in the three groups. Furthermore, there was no degeneration, necrosis, or accumulation of silver in the spinal cord on histology.
Conclusions: This study suggests that silver-hydroxyapatite-coated interbody cages produce good osteoconductivity and are not associated with direct neurotoxicity.
Keywords: hydroxyapatite; lumbar spinal interbody fusion; neurotoxicity; osteoconductivity; silver.
© 2022 The Authors. JOR Spine published by Wiley Periodicals LLC on behalf of Orthopaedic Research Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Martino ADI, Papalia R, Albo E, et al. Infection after spinal surgery and procedures. Eur Rev Med Pharmacol Sci. 2019;23(2):173‐178. - PubMed
-
- Pappou IP, Papadopoulos EC, Sama AA, Girardi FP, Cammisa FP. Postoperative infections in interbody fusion for degenerative spinal disease. Clin Orthop Relat Res. 2006;444:120‐128. - PubMed
LinkOut - more resources
Full Text Sources

