Mitochondrial TSPO Promotes Hepatocellular Carcinoma Progression through Ferroptosis Inhibition and Immune Evasion
- PMID: 36994647
- PMCID: PMC10214260
- DOI: 10.1002/advs.202206669
Mitochondrial TSPO Promotes Hepatocellular Carcinoma Progression through Ferroptosis Inhibition and Immune Evasion
Abstract
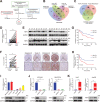
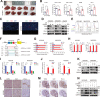
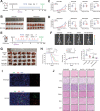
Hepatocellular carcinoma (HCC) is one of the most common malignancies with poor prognosis, and novel treatment strategies are urgently needed. Mitochondria are key regulators of cellular homeostasis and potential targets for tumor therapy. Here, the role of mitochondrial translocator protein (TSPO) in the regulation of ferroptosis and antitumor immunity is investigated and the potential therapeutic implications for HCC are assessed. TSPO is highly expressed in HCC and associated with poor prognosis. Gain- and loss-of-function experiments present that TSPO promotes HCC cell growth, migration, and invasion in vitro and in vivo. In addition, TSPO inhibits ferroptosis in HCC cells via enhancing the Nrf2-dependent antioxidant defense system. Mechanistically, TSPO directly interacts with P62 and interferes with autophagy, leading to the accumulation of P62. The P62 accumulation competes with KEAP1, preventing it from targeting Nrf2 for proteasomal degradation. Furthermore, TSPO promotes HCC immune escape by upregulating PD-L1 expression through Nrf2-mediated transcription. Notably, TSPO inhibitor PK11195 combines with anti-PD-1 antibody showing a synergistic anti-tumor effect in a mouse model. Overall, the results demonstrated that mitochondrial TSPO promotes HCC progression by inhibiting ferroptosis and antitumor immunity. Targeting TSPO can be a promising new strategy for HCC treatment.
Keywords: ferroptosis; hepatocellular carcinoma; immunotherapy; mitochondria; translocator protein.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., Bray F., Ca‐Cancer J. Clin. 2021, 71, 209. - PubMed
-
- Llovet J. M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J. F., de Oliveira A. C., Santoro A., Raoul J. L., Forner A., Schwartz M., Porta C., Zeuzem S., Bolondi L., Greten T. F., Galle P. R., Seitz J. F., Borbath I., Haussinger D., Giannaris T., Shan M., Moscovici M., Voliotis D., Bruix J., SHARP Investigators Study Group , N. Engl. J. Med.. 2008, 359, 378. - PubMed
-
- Kudo M., Finn R. S., Qin S., Han K. H., Ikeda K., Piscaglia F., Baron A., Park J. W., Han G., Jassem J., Blanc J. F., Vogel A., Komov D., Evans T. R. J., Lopez C., Dutcus C., Guo M., Saito K., Kraljevic S., Tamai T., Ren M., Cheng A. L., Lancet 2018, 391, 1163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- JBZX-202004/Health Commission of Zhejiang Province
- 82073144/National Natural Science Foundation of China
- 82003272/National Natural Science Foundation of China
- LHDMD22H310005/Huadong MedicineJoint Funds of the Zhejiang Provincial Natural Science Foundation of China
- 2019-I2M-5-030/Research Unit Project of Chinese Academy of Medical Sciences
LinkOut - more resources
Full Text Sources
Medical
Research Materials
