Autofluorescence imaging permits label-free cell type assignment and reveals the dynamic formation of airway secretory cell associated antigen passages (SAPs)
- PMID: 36994985
- PMCID: PMC10154029
- DOI: 10.7554/eLife.84375
Autofluorescence imaging permits label-free cell type assignment and reveals the dynamic formation of airway secretory cell associated antigen passages (SAPs)
Abstract
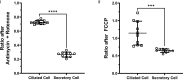
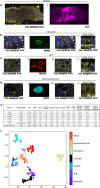
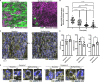
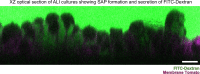
The specific functional properties of a tissue are distributed amongst its component cell types. The various cells act coherently, as an ensemble, in order to execute a physiologic response. Modern approaches for identifying and dissecting novel physiologic mechanisms would benefit from an ability to identify specific cell types in live tissues that could then be imaged in real time. Current techniques require the use of fluorescent genetic reporters that are not only cumbersome, but which only allow the study of three or four cell types at a time. We report a non-invasive imaging modality that capitalizes on the endogenous autofluorescence signatures of the metabolic cofactors NAD(P)H and FAD. By marrying morphological characteristics with autofluorescence signatures, all seven of the airway epithelial cell types can be distinguished simultaneously in mouse tracheal explants in real time. Furthermore, we find that this methodology for direct cell type-specific identification avoids pitfalls associated with the use of ostensibly cell type-specific markers that are, in fact, altered by clinically relevant physiologic stimuli. Finally, we utilize this methodology to interrogate real-time physiology and identify dynamic secretory cell associated antigen passages (SAPs) that form in response to cholinergic stimulus. The identical process has been well documented in the intestine where the dynamic formation of SAPs and goblet cell associated antigen passages (GAPs) enable luminal antigen sampling. Airway secretory cells with SAPs are frequently juxtaposed to antigen presenting cells, suggesting that airway SAPs, like their intestinal counterparts, not only sample antigen but convey their cargo for immune cell processing.
Keywords: FAD; NADH; airway epithelia; autofluorescence; cell biology; goblet cell associated antigen passages; mouse; secretory cell associated antigen passages.
Plain language summary
Imaging several cell types, at the same time, within a living tissue is no small endeavor. To do so, scientists usually have to perform genetic manipulations that make certain proteins in each cell type fluorescent and therefore easy to track. However, these approaches are cumbersome, limited, and often not applicable to intact human tissues. A possible alternative would be to make use of autofluorescence – the fact that certain molecules in living cells naturally fluoresce when exposed to a particular wavelength of light. For example, this is the case for NAD(P)H and FAD, two small molecules necessary for life’s biochemical processes, and whose intracellular levels and locations vary depending on cell type. In response, Shah, Hou et al. developed a new imaging technique that takes advantage of the unique autofluorescence signatures of NAD(P)H and FAD to distinguish between the seven different types of cells that line the surface of the airways of mice. Using their autofluorescence approach, Shah, Hou et al. were also able to discover a new role for secretory cells, which normally produce fluids, mucus and various proteins necessary for the lungs to work properly. The imaging experiments show that these cells could also sample material from the surface of the airway in a manner similar to how cells in the intestine take up material from the gut and pass their cargo to immune cells that mediate infection control or tolerance. Further studies should uncover more details about this new function of secretory lung cells. Other exciting discoveries may also emerge from researchers adopting the method developed by Shah, Hou et al. to examine a range of organs (both healthy and diseased), and attempting to apply it to human tissues.
© 2023, Shah, Hou et al.
Conflict of interest statement
VS, JH, VV, JX, MS, CL, JR No competing interests declared
Figures







Update of
- doi: 10.1101/2022.11.01.514675
Similar articles
-
Intestinal goblet cells sample and deliver lumenal antigens by regulated endocytic uptake and transcytosis.Elife. 2021 Oct 22;10:e67292. doi: 10.7554/eLife.67292. Elife. 2021. PMID: 34677124 Free PMC article.
-
An optimized method to visualize the goblet cell-associated antigen passages and identify goblet cells in the intestine, conjunctiva, and airway.Immunobiology. 2022 Nov;227(6):152260. doi: 10.1016/j.imbio.2022.152260. Epub 2022 Aug 17. Immunobiology. 2022. PMID: 36058107
-
In vivo labeling of epithelial cell-associated antigen passages in the murine intestine.Lab Anim (NY). 2020 Mar;49(3):79-88. doi: 10.1038/s41684-019-0438-z. Epub 2020 Feb 10. Lab Anim (NY). 2020. PMID: 32042160 Free PMC article.
-
Adverse immunological imprinting by cytomegalovirus sensitizing for allergic airway disease.Med Microbiol Immunol. 2019 Aug;208(3-4):469-473. doi: 10.1007/s00430-019-00610-z. Epub 2019 May 10. Med Microbiol Immunol. 2019. PMID: 31076879 Free PMC article. Review.
-
Goblet cell-associated antigen passage: A gatekeeper of the intestinal immune system.Immunology. 2023 Sep;170(1):1-12. doi: 10.1111/imm.13648. Epub 2023 Apr 17. Immunology. 2023. PMID: 37067238 Review.
Cited by
-
To label or not: the need for validation in label-free imaging.J Biomed Opt. 2024 Jun;29(Suppl 2):S22717. doi: 10.1117/1.JBO.29.S2.S22717. Epub 2024 Dec 20. J Biomed Opt. 2024. PMID: 39711795 Free PMC article. Review.
-
Airway hillocks are injury-resistant reservoirs of unique plastic stem cells.Nature. 2024 May;629(8013):869-877. doi: 10.1038/s41586-024-07377-1. Epub 2024 May 1. Nature. 2024. PMID: 38693267 Free PMC article.
-
Multispectral Imaging of Collagen, NAD(P)H and Flavin Autofluorescence in Mesenchymal Stem Cells Undergoing Trilineage Differentiation.Cells. 2024 Oct 18;13(20):1731. doi: 10.3390/cells13201731. Cells. 2024. PMID: 39451249 Free PMC article.
-
Single-cell transcriptomics of a dynamic cell behavior in murine airways.Elife. 2023 Apr 21;12:e76645. doi: 10.7554/eLife.76645. Elife. 2023. PMID: 37083555 Free PMC article.
References
-
- Deprez M, Zaragosi L-E, Truchi M, Becavin C, Ruiz García S, Arguel M-J, Plaisant M, Magnone V, Lebrigand K, Abelanet S, Brau F, Paquet A, Pe’er D, Marquette C-H, Leroy S, Barbry P. A single-cell atlas of the human healthy airways. American Journal of Respiratory and Critical Care Medicine. 2020;202:1636–1645. doi: 10.1164/rccm.201911-2199OC. - DOI - PubMed
-
- Dilipkumar A, Al-Shemmary A, Kreiß L, Cvecek K, Carlé B, Knieling F, Gonzales Menezes J, Thoma OM, Schmidt M, Neurath MF, Waldner M, Friedrich O, Schürmann S. Label-free multiphoton endomicroscopy for minimally invasive in vivo imaging. Advanced Science. 2019;6:1801735. doi: 10.1002/advs.201801735. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

